Abstract
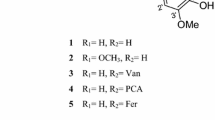
The present work summarizes results of isolation and identification of polar constituents of the methanolic extract of Ligustrum vulgare L. leaves and of the evaluation of inhibiting activity of selected isolates on rat lung cytosol fraction lipoxygenase. Six different compounds were isolated from the ethylacetate and butanol portions of the methanolic extract (hydroxytyrosol and its glucoside, ligustroflavon, oleuropein, acteoside, echinacoside). The inhibitory activity of oleuropein, echinacoside and the water infusion of Ligustrum vulgare leaves tested on LOX was expressed as IC50. Kinetic parameters (K M, V max) and type of inhibition were determined. As the most effective in competitive inhibition of LOX, oleuropein was proved.
Similar content being viewed by others
References
Bezáková, L., Grančai, D., Obložinská, I., Pauliková, I., Garaj, V., & Gáplovsky, M. (2007). Effect of flavonoids and cynarine from Cynara cardunculus L. on lipoxygenase activity. Acta Facultatis Pharmaceuticae Universitatis Comenianae, 54, 48–53.
Bezáková, L., Misik, V., Máleková, L., Svajdlenka, E., & Kostálová, D. (1996). Lipoxygenase inhibition and antioxidant properties of bisbenzylisoquinoline alkaloids isolated from Mahonia aquifolium. Pharmazie, 51, 758–761.
Bradford, M. M. (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. DOI: 10.1016/0003-2697(76)90527-3.
de la Puerta, R., Ruiz Gutierrez, V., & Hoult, J. R. S. (1999). Inhibition of leukocyte 5- lipoxygenase by phenolics from virgin olive oil. Biochemical Pharmacology, 57, 445–449. DOI: 10.1016/S0006-2952(98)00320-7.
Franzyk, H., Olsen, C. E., & Jansen, S. R. (2004). Dopaol 2-keto- and 2,3-diketoglycosides from Chelone obliqua. Journal of Natural Products, 67, 1052–1054. DOI: 10.1021/np0499416.
Hammermann, A. F., Damirov, J. A., & Sokolov, W. S. (1971). Einige aussichtsreiche Pflanzen der Volksmedizin von Azerbajdschan. Planta Medica, 20, 374–380. DOI: 10.1055/s-0028-1099719.
Hromádková, Z., Hirsch, J., & Ebringerová, A. (2010). Chemical evaluation of Fallopia species leaves and antioxidant properties of their non-cellulosic polysaccharides. Chemical Papers, 64, 663–672. DOI: 10.2478/s11696-010-0054-2.
Jiménez, J. T., O’Connell, S., Lyons, H., Bradley, B., & Hall, M. (2010). Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chemical Papers, 64, 434–442. DOI: 10.2478/s11696-010-0024-8.
Kemal, C., Louis-Flamberg, P., Krupinski-Olsen, R., & Shorter, A. L. (1987). Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity. Biochemistry, 26, 7064–7072. DOI: 10.1021/bi00396a031.
Kiss, A. K., Mańk, M., & Melzig, M. F. (2008). Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. Journal of Ethnopharmacology, 120, 220–225. DOI: 10.1016/j.jep.2008.08.015.
Kulkarni, A. P., Cai, Y., & Richards, I. S. (1992). Rat pulmonary lipoxygenase: dioxygenase activity and role of xenobiotic metabolism. International Journal of Biochemistry, 24, 255–261. DOI: 10.1016/0020-711X(92)90255-Y.
Ma, S.-C., He, Z.-D., Deng, X.-L., But, P. P.-H., Ooi, V. E.-C., Xu, H.-X., Lee, S. H.-S., & Lee, S.-F. (2001). In vitro evaluation of secoiridoid glucosides from the fruits of Ligustrum lucidum as antiviral agents. Chemical & Pharmaceutical Bulletin, 49, 1471–1473. DOI: 10.1248/cpb.49.1471.
Mabry, T. J., Markham, K. R., & Thomas, M. B. (1970). The systematic identification of flavonoids. New York, NY, USA: Springer-Verlag.
Mučaji, P., Nagy, M., Grančai, D., & Švajdlenka, E. (2006). Flavonoidné glykozidy Ligustrum vulgare L. Farmaceutický Obzor, 75(10–11), 266–271.
Nagao, T., Abe, F., & Okabe, H. (2001). Antiproliferative constituents in the plants 7. leaves of Clerodendron bungei and leaves and bark of C. trichotomum. Biological & Pharmaceutical Bulletin, 24, 1338–1342. DOI: 10.1248/bpb.24.1338.
Nagy, M., Križkovčaji, P., Kontšeková, Z., Šeršeň, F., & Krajčovič, J. (2009). Antimutagenic activity and radical scavenging activity of water infusions and phenolics from Ligustrum plants leaves. Molecules, 14, 509–518. DOI: 10.3390/molecules14010509.
Nagy, M., Spilková, J., Vrchovská, V., Kontšeková, Z., Šeršeň, F., Mučaji, P., & Grančai, D. (2006). Free radical scavenging activity of different extracts and some constituents from the leaves of Ligustrum vulgare and L. delavayanum. Fitoterapia, 77, 395–397. DOI: 10.1016/j.fitote.2006.04.010.
Pan, L. T., He, X. P., & Yanag, L. Y. (2002). Studies on chemical constituents in the leaf of Ligustrum delavayanum. Zhongguo Zhong Yao Za Zhi, 27, 754–756.
Pieroni, A., & Pachaly, P. (2000a). An ethnopharmacological study on common privet (Ligustrum vulgare) and phillyrea (Phillyrea latifolia). Fitoterapia, 71,Supplement 1, S89–S94. DOI: 10.1016/S0367-326X(00)00182-9.
Pieroni, A., & Pachaly, P. (2000b). Isolation and structure elucidation of ligustroflavone, a new apigenin triglycoside from the leaves Ligustrum vulgare L. Pharmazie, 55, 78–80.
Scogin, R. (1992). The distribution of acteoside among angiosperms. Biochemical Systematics and Ecology, 20, 477–480. DOI: 10.1016/0305-1978(92)90090-Z.
Shoemaker, M., Hamilton, B., Dairkee, S. H., Cohen, I., & Campbell, M. J. (2005). In vitro anticancer activity of twelve Chinese medicinal herbs. Phytotherapy Research, 19, 649–651. DOI: 10.1002/ptr.1702.
Stojanović-Radić, Z., Comić, L., Radulović, N., Dekić, M., Randelović, V., & Stefanović, O. (2010). Chemical composition and antimicrobial activity of Erodium species: E. ciconium L., E. cicutarium L., and E. absinthoides Willd. (Geraniaceae). Chemical Papers, 64, 368–377. 10.2478/s11696-010-0014-x.
Šeršeň, F., Mučaji, P., Grančai, D., Nagy, M., & Švajdlenka, E. (2006). Constituents of butanol extract from leaves of Ligustrum vulgare L. Acta Facultatis Pharmaceuticae Universitatis Comenianae, 53, 253–261.
Šmejkal, K., Babula, P., Šlapetová, T., Brognara, E., Dall’Acqua, S., Žemlička, M., Innocenti, G., & Cvačka, J. (2008). Cytotoxic activity of C-geranyl compounds from Paulownia tomentosa fruits. Planta Medica, 74, 1488–1491. DOI: 10.1055/s-2008-1081339.
Tattini, M., Galardi, C., Pinelli, P., Massai, R., Remorini, D., & Agati, G. (2004). Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytologist, 163, 547–561. DOI: 10.1111/j.1469-8137.2004.01126.x.
Wong, I. Y. F., He, Z.-D., Huang, Y., & Chen, Z.-Y. (2001). Antioxidative activities of phenylethanoid glycosides from Ligustrum purpurascens. Journal of Agricultural and Food Chemistry, 49, 3113–3119. DOI: 10.1021/jf0100604.
Yim, T. K., Wu, W. K., Pak, W. F., & Ko, K. M. (2001). Hepatoprotective action of an oleanolic acid-enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytotherapy Research, 15, 589–592. DOI: 10.1002/ptr.878.
Young, R. N. (1999). Inhibitors of 5-lipoxygenase: a therapeutic potential yet to be fully realized? European Journal of Medicinal Chemistry, 34, 671–685. DOI: 10.1016/S0223-5234(99)00225-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mučaji, P., Nagy, M., Záhradníková, A. et al. Polar constituents of Ligustrum vulgare L. and their effect on lipoxygenase activity. Chem. Pap. 65, 367–372 (2011). https://doi.org/10.2478/s11696-011-0015-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-011-0015-4