Abstract
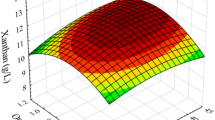
Gamma-linolenic acid (GLA, C18:3Δ6,9,12) is an n-6 polyunsaturated fatty acid (PUFA) that has been used for the alleviation and treatment of a number of symptoms and diseases. Increasing GLA demand has led to a search for alternative producers and potential strategies for GLA production. Based on the successful performance of Hansenula polymorpha, a methylotrophic yeast, as a “cell factory” for the production of valuable bioproducts, a bioprocess development approach was implemented for GLA production in the recombinant yeast carrying the mutated Δ6-desaturase gene of Mucor rouxii. Using a substrate-feeding strategy under glycerol-limited conditions, the physical-chemical variables during the fed-batch fermentation of the recombinant H. polymorpha were optimised for GLA production through response surface methodology using a Box-Behnken design. The medium composition, including yeast extract and trace elements, and dissolved oxygen tension (DOT) were targeted. We found that DOT was the most effective variable for enhancing GLA yield. These results also suggest that the optimum conditions for GLA production are 28 % saturation of DOT, 1 g L−1 of yeast extract and 3.6 mL L−1 of the Pichia trace metals 1 (PTM1).
Similar content being viewed by others
References
Ahmed, S. U., Singh, K. S., Pandey, A., Kanjilal, S., & Prasad, R. B. N. (2009). Application of response surface method for studying the role of dissolved oxygen and agitation speed on gamma-linolenic acid production. Applied Biochemistry and Biotechnology, 152, 108–116. DOI: 10.1007/s12010-008-8256-6.
Ahmed, S. U., Singh, S. K., Pandey, A., Kanjilal, S., & Prasad, R. B. N. (2008). Fatty acid profiling during microbial lipid production under varying pO2 and impeller tip speeds. Applied Biochemistry and Biotechnology, 151, 599–609. DOI: 10.1007/s12010-008-8261-9.
Certik, M., Megova, J., & Horenitzky, R. (1999). Effect of nitrogen sources on the activities of lipogenic enzymes in oleaginous fungus Cunninghamella echinulata. The Journal of General and Applied Microbiology, 45, 289–293. DOI: 10.2323/jgam.45.289.
Certik, M., & Shimizu, S. (1999). Biosynthesis and regulation of microbial polyunsaturated fatty acid production. Journal of Bioscience and Bioengineering, 87, 1–14. DOI: 10.1016/S1389-1723(99)80001-2.
Chen, H.-C., & Chang, C.-C. (1996). Production of γ-linolenic acid by the fungus Cunninghamella echinulata CCRC 31840. Biotechnology Progress, 12, 338–341. DOI: 10.1021/bp9600 09y.
Chen, P. T., Chiang, C.-J., & Chao, Y.-P. (2010). Medium optimization and production of secreted Renilla luciferase in Bacillus subtilis by fed-batch fermentation. Biochemical Engineering Journal, 49, 395–400. DOI: 10.1016/j.bej.2010.02.001.
Chen, Z., Wang, Z., He, X., Guo, X., Li, W., & Zhang, B. (2008). Uricase production by a recombinant Hansenula polymorpha strain harboring Candida utilis uricase gene. Applied Microbiology and Biotechnology, 79, 545–554. DOI: 10.1007/s00253-008-1472-8.
d’Anjou, M. C., & Daugulis, A. J. (1997). A model-based feeding strategy for fed batch fermentation of recombinant Pichia pastoris. Biotechnology Techniques, 11, 865–868. DOI: 10.1023/A:1018449930343.
du Preez, J. C., Immelman, M., Kock, J. L. F., & Kilian, S. G. (1997). The effect of acetic acid concentration on the growth and production of gamma-linolenic acid by Mucor circinelloides CBS 203.28 in fed-batch culture. World Journal of Microbiology and Biotechnology, 13, 81–87. DOI: 10.1007/BF02770812.
Dyal, S. D., Bouzidi, L., & Narine, S. S. (2005). Maximizing the production of γ-linolenic acid in Mortierella ramanniana var. ramammiana as a function of pH, temperature and carbon source, nitrogen source, metal ions and oil supplementation. Food Research International, 38, 815–829. DOI: 10.1016/j.foodres.2005.04.002.
Fakas, S., Čertik, M., Papanikolaou, S., Aggelis, G., Komaitis, M., & Galiotou-Panagotou, M. (2008). γ-Linolenic acid production by Cunninghamella echinulata growing on complex organic nitrogen sources. Bioresource Technology, 99, 5986–5990. DOI: 10.1016/j.biortech.2007.10.016.
Gellissen, G., Kunze, G., Gaillardin, C., Cregg, J. M., Berardi, E., Veenhuis, M., & van der Klei, I. (2005). New yeast expression platforms based on methylotrophic Hansenula polymorpha and Pichia pastoris and on dimorphicArxula adeninivorans and Yarrowia lipolytica — A comparison. FEMS Yeast Research, 5, 1079–1096. DOI: 10.1016/j.femsyr.2005.06.004.
Gill, I., & Valivety, R. (1997). Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends in Biotechnology, 15, 401–409. DOI: 10.1016/S0167-7799(97)01076-7.
Guilmanov, V., Ballistreri, A., Impallomeni, G., & Gross, R. A. (2002). Oxygen transfer rate and sophorose lipid production by Candida bombicola. Biotechnology and Bioengineering, 77, 489–494. DOI:10.1002/bit.10177.
Hansson, L., & Dostálek, M. (1988). Effect of culture conditions on mycelial growth and production of γ-linolenic acid by the fungus Mortierella ramanniana. Applied Microbiology and Biotechnology, 28, 240–246. DOI: 10.1007/BF00250448.
Higashiyama, K., Murakami, K., Tsujimura, H., Matsumoto, N., & Fujikawa, S (1999). Effects of dissolved oxygen on the morphology of an arachidonic acid production by Mortierella alpina 1S-4. Biotechnology and Bioengineering, 63, 442–448. DOI: 10.1002/(SICI)1097-0290(19990520)63:4〈442::AIDBIT7〉 3.0.CO;2-9.
Hiruta, O., Futamura, T., Takebe, H., Satoh, A., Kamisaka, Y., Yokochi, T., Nakahara, T., & Suzuki, O. (1996). Optimization and scale-up of γ-linolenic acid production by Mortierella ramanniana MM 15-1, a high γ-linolenic acid producing mutant. Journal of Fermentation and Bioengineering, 82, 366–370. DOI: 10.1016/0922-338X(96)89152-5.
Jahic, M., Veide, A., Charoenrat, T., Teeri, T., & Enfors, S.-O. (2006). Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnology Progress, 22, 1465–1473. DOI: 10.1021/bp060171t.
Jang, H.-D., Lin, Y.-Y., & Yang, S.-S. (2005). Effect of culture media and conditions on polyunsaturated fatty acids production by Mortierella alpina. Bioresource Technology, 96, 1633–1644. DOI: 10.1016/j.biortech.2004.12.027.
Laoteng, K., Ruenwai, R., Tanticharoen, M., & Cheevadhanarak, S. (2005). Genetic modification of essential fatty acids biosynthesis in Hansenula polymorpha. FEMS Microbiology Letters, 245, 169–178. DOI: 10.1016/j.femsle.2005.03.006.
Lepage, G., & Roy, C. C. (1984). Improved recovery of fatty acid through direct transesterification without prior extraction or purification. Journal of Lipid Research, 25, 1391–1396.
Mamatha, S. S., Ravi, R., & Venkateswaran, G. (2008). Medium optimization of gamma linolenic acid production in Mucor rouxii CFR-G15 using RSM. Food and Bioprocess Technology, 1, 405–409. DOI: 10.1007/s11947-008-0103-9.
Myers, R. H., & Montgomery, D. C. (2002). Response surface methodology: Process and product optimization using designed experiments. New York, NY, USA: Wiley.
Na-Ranong, S., Laoteng, K., Kittakoop, P., Tanticharoen, M., & Cheevadhanarak, S. (2006). Targeted mutagenesis of a fatty acid Δ6-desaturase from Mucor rouxii: Role of amino acid residues adjacent to histidine-rich motif II. Biochemical and Biophysical Research Communications, 339, 1029–1034. DOI: 10.1016/j.bbrc.2005.11.115.
Nasrabadi, N. M. R., & Razavi, S. H. (2010). Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. Journal of Bioscience and Bioengineering, 109, 361–368. DOI: 10.1016/j.jbiosc.2009.10.013.
Papanikolaou, S., Komaitis, M., & Aggelis, G. (2004). Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresource Technology, 95, 287–291. DOI: 10.1016/j.biortech.2004.02.016.
Plantz, B. A., Nickerson, K., Kachman, S. D., & Schlegel, V. L. (2007). Evaluation of metals in a defined medium for Pichia pastoris expressing recombinant β-galactosidase. Biotechnology Progress, 23, 687–692. DOI: 10.1021/bp060332t.
Ratledge, C. (2004). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie, 86, 807–815. DOI: 10.1016/j.biochi.2004.09.017.
Ratledge, C., & Wynn, J. P. (2002). The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Advances in Applied Microbiology, 51, 1–44. DOI: 10.1016/S0065-2164(02)51000-5.
Shen, Y., Yuan, W., Pei, Z., & Mao, E. (2010). Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Applied Biochemistry and Biotechnology, 160, 1674–1684. DOI: 10.1007/s12010-009-8659-z.
StatSoft Inc. (2007). Statistica, Version 8. Tulsa, OK, USA: StatSoft Inc.
Wu, S.-T., Yu, S.-T., & Lin, L.-P. (2005). Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochemistry, 40, 3103–3108. DOI: 10.1016/j.procbio.2005.03.007.
Zhao, X., Kong, X., Hua, Y., Feng, B., & Zhao, Z. K. (2008). Medium optimization for lipid production through co-fermentation of glucose and xylose by oleaginous yeast Lipomyces starkeyi. European Journal of Lipid Science and Technology, 110, 405–412. DOI: 10.1002/ejlt.200700224.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khongto, B., Laoteng, K. & Tongta, A. Enhancing the production of gamma-linolenic acid in Hansenula polymorpha by fed-batch fermentation using response surface methodology. Chem. Pap. 65, 124–131 (2011). https://doi.org/10.2478/s11696-010-0099-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0099-2