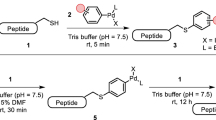
Abstract
Biological studies showed that assembles of biomolecules can dramatically change their physiological effectiveness. Covalent coupling of different types of biomolecules leads to novel biomacromolecules of different properties. Generally, bioconjugate chemistry opens a new dimension in biomedical and biotechnology research. In this review, some important chemical methods of bioconjugates preparation used in the practice are described. Proteins and saccharides modification methods and employment of linkers used to achieve new functionalities are discussed. Common bioconjugation methods are emphasized and novel methods from recent years are described. Except in chemistry, benefits and limits of the studied methods are outlined.
Similar content being viewed by others
References
Albericio, F. (2004). Developments in peptide and amide synthesis. Current Opinion in Chemical Biology, 8, 211–221. DOI: 10.1016/j.cbpa.2004.03.002.
Amir-Kroll, H., Nussbaum, G., & Cohen, I. R. (2003). Proteins and their derived peptides as carriers in a conjugate vaccine for Streptococcus pneumoniae: Self-heat shock protein 60 and tetanus toxoid. The Journal of Immunology, 170, 6165–6171.
Anderson, P., Pichichero, M. E., & Insel, R. A. (1985). Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or CRM197. The Journal of Clinical Investigation, 76, 52–59. DOI: 10.1172/JCI111976.
Baskin, J. M., Prescher, J. A., Laughlin, S. T., Agard, N. J., Chang, P. V., Miller, I, A., Lo, A., Codelli, J. A., & Bertozzi, C. R. (2007). Copper-free click chemistry for dynamic in vivo imaging. Proceedings of the National Academy of Sciences, 104, 16793–16797. DOI: 10.1073/pnas.0707090104.
Bauminger, S., & Wilcheck, M. (1980). The use of carbodiimides in the preparation of immunizing conjugates. Methods in Enzymology, 70, 151–159.
Berkin, A., Coxon, B., & Pozsgay, V. (2002). Towards a synthetic glycoconjugate vaccine against Neisseria meningitidis A. Chemistry — A European Journal, 8, 4424–4433. DOI: 10.1002/1521-3765(20021004)8:19<4424::AID-CHEM4424>3.0.CO;2-1.
Bernstein, M. A., & Hall, L. D. (1980). A general synthesis of model glycoproteins: coupling of alkenyl glycosides to proteins, using reductive ozonolysis followed by reductive amination with sodium cyanoborohydride. Carbohydrate Research, 78, C1–C3. DOI: 10.1016/S0008-6215(00)83676-9.
Bode, J. W., Fox, R. M., & Baucom, K. D. (2006). Chemoselective amide ligations by decarboxylative condensations of N-alkylhydroxylamines and α-ketoacids. Angewandte Chemie International Edition, 45, 1248–1252. DOI: 10.1002/anie.200503991.
Boratyńsky, J., & Roy, R. (1998). High temperature conjugation of proteins with carbohydrates. Glycoconjugate Journal, 15, 131–138. DOI: 10.1023/A:1007067513242.
Bulpitt, P., & Aeschlimann, D. (1999). New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. Journal of Biomedical Materials Research Part A, 47, 152–169. DOI: 10.1002/(SICI)1097-4636(199911)47:2<152::AID-JBM5>3.0.CO;2-I.
Bystrický, S., Machová, E., Bartek, P., Kolarova, N., & Kogan, G. (2000). Conjugation of yeast mannans with protein employing cyanopyridinium agent (CDAP). an effective route of antifungal vaccine preparation. Glycoconjugate Journal, 17, 677–680. DOI: 10.1023/A:1011002118819.
Canalle, L. A., Löwik, D. W. P. M., & Hest, J. C. M. (2010). Polypeptide-polymer bioconjugates. Chemical Society Reviews, 39, 329–353, DOI: 10.1039/b807871h.
Chan, T. R., Hilgraf, R., Sharpless, K. B., & Fokin, V. V. (2004). Polytriazoles as copper(I)-stabilizing ligands in catalysis. Organic Letters, 6, 2853–2855. DOI: 10.1021/ol0493094.
Dalpathado, D. S., Jiang, H., Kater, M. A., & Desaire, H. (2005). Reductive amination of carbohydrates using NaBH(OAc)3. Analytical and Bioanalytical Chemistry, 381, 1130–1137. DOI: 10.1007/s00216-004-3028-9.
Dawson, P. E., Muir, T. W., Clark-Lewis, I., & Kent, S. B. (1994). Synthesis of proteins by native chemical ligation. Science, 266, 776–779. DOI: 10.1126/science.7973629.
Ďurana, R., Lacík, I., Paulovičová, E., & Bystický, S. (2006). Functionalization of mannans from pathogenic yeasts by different means of oxidations—preparation of precursors for conjugation reactions with respect to preservation of immunological properties. Carbohydrate Polymers, 63, 72–81. DOI: 10.1016/j.carbpol.2005.08.003.
Dziadek, S., Jacques, S., & Bundle, D. R. (2008). A novel linker methodology for the synthesis of tailored conjugate vaccines composed of complex carbohydrate antigens and specific THcell peptide epitopes. Chemistry — A European Journal, 14, 5908–5917. DOI: 10.1002/chem.200800065.
Farkaš, P., & Bystrický, S. (2008). Hydrolysis of the terminal dimethylacetal moiety on the spacers bound to carboxy groups containing glucans. Carbohydrate Polymers, 74, 133–136. DOI: 10.1016/j.carbpol.2008.01.005.
Farkaš, P., & Bystický, S. (2007). Efficient activation of carboxyl polysaccharides for the preparation of conjugates. Carbohydrate Polymers, 68, 187–190. DOI: 10.1016/j.carbpol.2006.07.013.
García, A., Hernández, K., Chico, B., García, D., Villalonga, M. L., & Villalonga, R. (2009). Preparation of thermostable trypsin.polysaccharide neoglycoenzymes through Ugi multicomponent reaction. Journal of Molecular Catalysis B: Enzymatic, 59, 126–130. DOI: 10.1016/j.molcatb.2009.02.001.
Grabarek, Z., & Gergely, J. (1990). Zero-length crosslinking procedure with the use of active esters. Analytical Biochemistry, 185, 131–135. DOI: 10.1016/0003-2697(90)90267-D.
Grandjean, C., Boutonnier, A., Dassy, B., Fournier, J.-M., & Mulard, L. A. (2009). Investigation towards bivalent chemically defined glycoconjugate immunogens prepared from acid-detoxified lipopolysaccharide of Vibrio cholerae O1, serotype Inaba. Glycoconjugate Journal, 26, 41–55. DOI: 10.1007/s10719-008-9160-6.
Grandjean, C., Boutonnier, A., Guerreiro, C., Fournier, J.-M., & Mulard, L. A. (2005). On the preparation of carbohydrate-protein conjugates using the traceless Staudinger ligation. Journal of the Organic Chemistry, 70, 7123–7132. DOI: 10.1021/jo0505472.
Griesbaum, K. (1970). Problems and possibilities of the free-radical addition of thiols to unsaturated compounds. Angewandte Chemie International Edition, 9, 273–287. DOI: 10.1002/anie.197002731.
Gurd, F. R. N. (1967). Carboxymethylation. Methods in Enzymology, 11, 532–541. DOI: 10.1016/S0076-6879(67)11064-1.
Hermanson, G. T. (1996). Bioconjugate techniques. San Diego, CA, USA: Academic Press, Inc.
Hou, S.-J., Saksena, R., & Kova., P. (2008). Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydrate Research, 343, 196–210. DOI: 10.1016/j.carres.2007.10.015.
Hoyle, C. E., & Bowman, C. N. (2010). Thiol-ene click chemistry. Angewandte Chemie International Edition, 49, 1540–1573. DOI: 10.1002/anie.200903924.
Izumi, M., Okumura, S., Yuasa, H., & Hashimoto, H. (2003). Mannose-BSA conjugates: Comparison between commercially available linkers in reactivity and bioactivity. Journal of Carbohydrate Chemistry, 22, 317–329. DOI: 10.1081/CAR-120023475.
Johnson, E. C. B., & Kent, S. B. H. (2006). Insights into the mechanism and catalysis of the native chemical ligation reaction. Journal of the American Chemical Society, 128, 6640–6646. DOI: 10.1021/ja058344i.
Jonkheijm, P., Weinrich, D., Köhn, M., Engelkamp, H., Christianen, P. C. M., Kuhlmann, J., Maan, J. C., Nüsse, D., Schroeder, H., Wacker, R., Breinbauer, R., Niemeyer, C. M., & Waldmann, H. (2008). Photochemical surface patterning by the thiol-ene reaction. Angewandte Chemie International Edition, 47, 4421–4424. DOI: 10.1002/anie.200800101.
Kiick, K. L., Saxon, E., Tirrell, D. A., & Bertozzi, C. R. (2002). Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proceedings of the National Academy of Sciences, 99, 19–24. DOI: 10.1073/pnas.012583299.
Kohn, J., & Wilchek, M. (1983). 1-Cyano-4-dimethylamino pyridinium tetrafluoroborate as a cyanylating agent for the covalent attachment of ligand to polysaccharide resins. FEBS Letters, 154, 209–210. DOI: 10.1016/0014-5793(83)80905-3.
Köhn, M., & Breinbauer, R. (2004). The Staudinger ligation — a gift to chemical biology. Angewandte Chemie International Edition, 43, 3106–3116. DOI: 10.1002/anie.200401744.
Kubler-Kielb, J., Liu, T.-Y., Mocca, C., Majadly, F., Robbins, J. B., & Schneerson, R. (2006). Additional conjugation methods and immunogenicity of Bacillus anthracis poly-γ-D-glutamic acid-protein conjugates. Infection and Immunity, 74, 4744–4749. DOI: 10.1128/IAI.00315-06.
Kubler-Kielb, J., & Pozsgay, V. (2005). A new method for conjugation of carbohydrates to proteins using an aminooxy-thiol heterobifunctional linker. Journal of the Organic Chemistry, 70, 6987–6990. DOI: 10.1021/jo050934b.
Lees, A., Sen, G., & Acosta, A. L. (2006). Versatile and efficient synthesis of protein.polysaccharide conjugate vaccines using aminooxy reagents and oxime chemistry. Vaccine, 24, 716–729. DOI: 10.1016/j.vaccine.2005.08.096.
Leung, C., Chibba, A., Gómez-Biagi, R. F., & Nitz, M. (2009). Efficient synthesis and protein conjugation of β-(1→6)-D-N-acetylglucosamine oligosaccharides from the polysaccharide intercellular adhesin. Carbohydrate Research, 344, 570–575. DOI: 10.1016/j.carres.2008.12.021.
Li, C.-J. (2005). Organic reactions in aqueous media with a focus on carbon.carbon bond formations: A decade update. Chemical Reviews, 105, 3095–3165. DOI: 10.1021/cr030009u.
Lin, F. L., Hoyt, H. M., van Halbeek, H., Bergman, R. G., & Bertozzi, C. R. (2005). Mechanistic investigation of the Staudinger ligation. Journal of the American Chemical Society, 127, 2686–2695. DOI: 10.1021/ja044461m.
Lutz, J.-F., & Börner, H. G. (2008). Modern trends in polymer bioconjugates design. Progress in Polymer Science, 33, 1–39. DOI: 10.1016/j.progpolymsci.2007.07.005.
Lutz, J.-F., & Zarafshani, Z. (2008). Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide.alkyne “click” chemistry. Advanced Drug Delivery Reviews, 60, 958–970. DOI: 10.1016/j.addr.2008.02.004.
Mieszala, M., Kogan, G., & Jennings, H. J. (2003). Conjugation of meningococcal lipooligosaccharides through their lipid A terminus conserves their inner epitopes and results in conjugate vaccines having improved immunological properties. Carbohydrate Research, 338, 167–175. DOI: 10.1016/S0008-6215(02)00395-6.
Montalbetti, C. A. G. N., & Falque, V. (2005). Amide bond formation and peptide coupling. Tetrahedron, 61, 10827–10852. DOI: 10.1016/j.tet.2005.08.031.
Pavliakova, D., Chu, C., Bystrický, S., Tolson, N. W., Shiloach, J., Kaufman, J. B., Bryla, D. A., Robbins, J. B., & Schneerson, R. (1999). Treatment with succinic anhydride improves the immunogenicity of Shigella flexneri Type 2a O-specific polysaccharide-protein conjugates in mice. Infection and Immunity, 67, 5526–5529.
Pawlowski, A., Källenius, G., & Svenson, S. B. (2000). Preparation of pneumococcal capsular polysaccharide-protein conjugate vaccines utilizing new fragmentation and conjugation technologies. Vaccine, 18, 1873–1885. DOI: 10.1016/S0264-410X(99)00336-9.
Pawlowski, A., Källenius, G., & Svenson, S. B. (1999). A new method of non-cross-linking conjugation of polysaccharides to proteins via thioether bonds for the preparation of saccharide.protein conjugate vaccines. Vaccine, 17, 1474–1483. DOI: 10.1016/S0264-410X(98)00385-5.
Pozsgay, V., & Kubler-Kielb, J. (2008). Conjugation methods toward synthetic vaccines. In R. Roy (Ed.), Carbohydrate based vaccines (pp. 36–70). Washington, DC, USA: American Chemical Society.
Pozsgay, V., Vieira, N. E., & Yergey, A. (2002). A method for bioconjugation of carbohydrates using Diels-Alder cycloaddition. Organic Letters, 4, 3191–3194. DOI: 10.1021/ol026179v.
Rideout, D. C., & Breslow, R. (1980). Hydrophobic acceleration of Diels-Alder reactions. Journal of the American Chemical Society, 102, 7816–7817. DOI: 10.1021/ja00546a048.
Rodionov, V. O., Presolski, S. I., Gardinier, S., Lim, Y.-H., & Finn, M. G. (2007). Benzimidazole and related ligands for Cu-catalyzed azide-alkyne cycloaddition. Journal of the American Chemical Society, 129, 12696–12704. DOI: 10.1021/ja072678l.
Rogers, L. K., Leinweber, B. L., & Smith, C. V. (2006). Detection of reversible protein thiol modifications in tissues. Analytical Biochemistry, 358, 171–184. DOI: 10.1016/j.ab.2006.08.020.
Rostovtsev, V. V., Green, L. G., Fokin, V. V., & Sharpless, K. B. (2002). A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angewandte Chemie International Edition, 41, 2596–2599.DOI: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4.
Sanki, A. K., Talan, R. S., & Sucheck, S. J. (2009). Synthesis of small glycopeptides by decarboxylative condensation and insight into the reaction mechanism. The Journal of Organic Chemisty, 74, 1886–1896. DOI: 10.1021/jo802278w.
Saxon, E., & Bertozzi, C. R. (2000). Cell surface engineering by a modified Staudinger reaction. Science, 287, 2007–2010. DOI: 10.1126/science.287.5460.2007.
Schlottmann, S. A., Jain, N., Chirmule, N., & Esser, M. T. (2006). A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. Journal of Immunological Methods, 309, 75–85. DOI: 10.1016/j.jim.2005.11.019.
Shafer, D. E., Toll, B., Schuman, R. F., Nelson, B. L., Mond, J. J., & Lees, A. (2000). Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) for use in protein-polysaccharide conjugate vaccines and immunological reagents. II. Selective crosslinking of proteins to CDAP-activated polysaccharides. Vaccine, 18, 1273–1281. DOI: 10.1016/S0264-410X(99)00370-9.
Singh, R., & Whitesides, G. M. (1991). A reagent for reduction of disulfide bonds in proteins that reduces disulfide bonds faster than does dithiothreitol. Journal of Organic Chemistry, 56, 2332–2337. DOI: 10.1021/jo00007a018.
Sletten, E. M., & Bertozzi, C. R. (2009). Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angewandte Chemie International Edition, 48, 6974–6998. DOI: 10.1002/anie.200900942.
Sletten, E. M., & Bertozzi, C. R. (2008). A hydrophilic azacyclooctyne for Cu-free click chemistry. Organic Letters, 10, 3097–3099. DOI: 10.1021/ol801141k.
Soellner, M. B., Nilsson, B. L., & Raine, R. T. (2006). Reaction mechanism and kinetics of the traceless Staudinger ligation. Journal of the American Chemical Society, 128, 8820–8828. DOI: 10.1021/ja060484k.
Staudinger, H., & Meyer, J. (1919). Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und phosphinimine. Helvetica Chimimica Acta, 2, 635–646. DOI: 10.1002/hlca.19190020164.
Tietze, L. F., Schröter, C., Gabius, S., Brinck, U., Goerlach-Graw, A., & Gabius, H.-J. (1991). Conjugation of paminophenyl glycosides with squaric acid diester to a carrier protein and the use of the neoglycoprotein in the histochemical detection of lectins. Bioconjugate Chemistry, 2, 148–153. DOI: 10.1021/bc00009a003.
Wang, J. Y., Chang, A. H. C., Guttormsen, H.-K., Rosas, A. L., & Kaspe, D. L. (2002). Construction of designer glycoconjugate vaccines with size-specific oligosaccharide antigens and site-controlled coupling. Vaccine, 21, 1112–1117. DOI: 10.1016/S0264-410X(02)00625-4.
Wang, Q., Chan, T. R., Hilgraf, R., Fokin, V. V., Sharpless, K. B., & Finn, M. G. (2003). Bioconjugation by copper(I)-catalyzed azide-alkine [3 + 2] cycloaddition. Journal of the American Chemical Society, 125, 3192–3193. DOI: 10.1021/ja021381e.
Xin, H., Dziadek, S., Bundle, D. R., & Cutler, J. E. (2008). Synthetic glycopeptide vaccines combining β-mannan and peptide epitopes induce protection against candidiasis. Proceedings of the National Academy of Sciences, 105, 13526–13531. DOI: 10.1073/pnas.0803195105.
Xue, J., Pan, Y., & Guo, Z. (2002). Neoglycoprotein cancer vaccines: synthesis of an azido derivative of GM3 and its efficient coupling to proteins through a new linker. Tetrahedron Leters, 43, 1599–1602. DOI: 10.1016/S0040-4039(02)00071-0.
Zhang, J., Yergey, A., Kowalak, J., & Kováč, P. (1998). Linking carbohydrates to proteins using N-(2,2-dimethoxyethyl)-6-hydroxy hexanamide. Tetrahedron, 54, 11783–11792. DOI: 10.1016/S0040-4020(98)83039-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farkaš, P., Bystrický, S. Chemical conjugation of biomacromolecules: A mini-review. Chem. Pap. 64, 683–695 (2010). https://doi.org/10.2478/s11696-010-0057-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0057-z