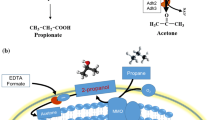
Abstract
Methanol has recently attracted significant interest in the energetic field. Current technology for the conversion of methane to methanol is based on energy intensive endothermic steam reforming followed by catalytic conversion into methanol. The one-step method performed at very low temperatures (35°C) is methane oxidation to methanol via bacteria. The aim of this work was to examine the role of copper in the one-step methane oxidation to methanol by utilizing whole cells of Methylosinus trichosporium OB3b bacteria. From the results obtained it was found that copper concentration in the medium influences the rate of bacterial biomass growth or methanol production during the process of methane oxidation to methanol. The presented results indicate that the process of methane oxidation to methanol by Methylosinus trichosporium OB3b bacteria is most efficient when the mineral medium contains 1.0 × 10−6 mol dm−3 of copper. Under these conditions, a satisfactory growth of biomass was also achieved.
Similar content being viewed by others
References
Barta, T. M., & Hanson, R. S. (1993). Genetics of methane and methanol oxidation in Gram-negative methylotrophic bacteria. Antonie van Leeuwenhoek, 64, 109–120. DOI: 10.1007/BF00873021.
Bender, M., & Conrad, R. (1995). Effect of CH4 concentrations and soil conditions on the induction of CH4 oxidation activity. Soil Biology & Biochemistry, 27, 1517–1527. DOI: 10.1016/0038-0717(95)00104-M.
Benstead, J., King, G. M., & Williams, H. G. (1998). Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Applied Environmental Microbiology, 64, 1091–1098. DOI: 0099-2240/98/$04.00+0.
Berndt, H., Martin, A., Bruckner, A., Schreier, E., Müller, D., Kosslick, H., Wolf, G.-U., & Lücke B. (2000). Structure and catalytic properties of VOx/MCM materials for the partial oxidation of methane to formaldehyde. Journal of Catalysis, 191, 384–400. DOI: 10.1006/jcat.1999.2786.
Brown, M. J., & Parkyns, N. D. (1991). Progress in the partial oxidation of methane to methanol and formaldehyde. Catalysis Today, 8, 305–335. DOI: 10.1016/0920-5861(91)80056-F.
Burch, R., Squire, G. D., & Tsang, S. C. (1989). Direct conversion of methane into methanol. Journal of the Chemical Society, Faraday Transactions 1,85, 3561–3568. DOI: 10.1039/F19898503561.
Dalton, H., Priori, S. D., Leak, D. J., & Stanley, S. H. (1984). Regulation and control of methane monooxygenase. In R. L. Crawford and R. S. Hanson (Eds.), Microbial growth on C 1 compounds (pp. 75–82). Washington, D.C.: American Society for Microbiology.
Feng, W., Knopf, F. C., & Dooley, K. M. (1994). Effects of pressure, third bodies, and temperature profiling on the noncatalytic partial oxidation of methane. Energy & Fuels, 8, 815–1005. DOI: 10.1021/ef00046a001.
Foulds, G. A., Gray, B. F, Miller, S. A., & Walker, G. S. (1993). Homogeneous gas-phase oxidation of methane using oxygen as oxidant in an annular reactor. Industrial & Engineering Chemistry Research, 32, 780–787. DOI: 10.1021/ie00017a003.
Furuto, T., Takeguchi, M., & Okura, I. (1999). Semicontinuous methanol biosynthesis by Methylosinus trichosporium Ob3b. Journal of Molecular Catalysis A: Chemical, 144, 257–261. DOI: 10.1016/S1381-1169(99)00007-2.
Haggin, J. (1990). Alternative fuels to petroleum gain increased attention. Chemical & Engineering News, 23, 25–27.
Hanson, R. S., & Hanson, T. E. (1996). Methanotrophic bacteria. Microbiology Review, 60, 439–471.
Harwood, J. H., & Pirt, S. J. (1972). Quantitative aspects of growth of the methane oxidizing bacterium Methylococcus capsulatus on methane in shake flask and continuous chemostat culture. Journal of Applied Bacteriology, 35, 597–607. DOI: 10.1111/j.1365-2672.1972.tb03741.x.
Hunter, N. R., Gesser, H. D., Morton, L. A., Yarlagadda, P. S., & Fung, D. P. C. (1990). Methanol formation at high pressure by the catalyzed oxidation of natural gas and by the sensitized oxidation of methane. Applied Catalysis A: General, 57, 45–54. 10.1016/S0166-9834(00)80722-8.
Kudo, H., & Ono, T. (1997). Partial oxidation of CH4 over ZSM-5 catalysts. Applied Surface Science, 121–122. 413–416. DOI: 10.1016/S0169-4332(97)00348-6.
Lamb, S. C., & Garver, J. C. (1980). Batch- and continuous-culture studies of methane-utilizing mixed culture. Biotechnology & Bioengineering, 22, 2097–2118. DOI: 10.1002/bit.260221009.
Lieberman, R. L., & Rosenzweig, A. C. (2004). Biological methane oxidation: regulation, biochemistry and active site structure of particulate methane monooxygenase. Critical Reviews in Biochemistry and Molecular Biology, 39, 147–164. DOI: 10.1080/10409230490475507.
Lipscomb, J. D. (1996). Biochemistry of the soluble methane monooxygenase. Annual Review of Microbiology, 48, 371–399. DOI: 10.1146/annurev.mi.48.100194.002103.
Maślakiewicz, P., & Steczko J. (1989). Monooksygenaza metanowa — występowanie, właściwości oraz perspektywy wykorzystania w procesach biotechnologicznych. Biotechnologia, 3(4), 56–61.
Michalkiewicz, B. (2004). Partial oxidation of methane to formaldehyde and methanol using molecular oxygen over Fe-ZSM-5. Applied Catalysis A: General, 277, 147–153. DOI: 10.1016/j.apcata.2004.09.005.
Otsuka, K., & Hatano, M. (1987). The catalysts for the synthesis of formaldehyde by partial oxidation of methane. Journal of Catalysis, 108, 252–255. DOI: 10.1016/0021-9517(87)90172-2.
Otsuka, K., & Wang, Y. (2001). Direct conversion of methane into oxygenates. Applied Catalysis A: General, 222, 145–161. DOI: 10.1016/S0926-860X(01)00837-7.
Patt, T. E., Cole, G. C., Bland, J., & Hanson, R. S. (1974). Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. Journal of Bacteriology, 120, 955–964.
Periana, R. A., Taube, D. J., Gamble, S., Taube, H., Satoh, T., & Fujii, H. (1998). Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science, 280, 560–564. DOI: 10.1126/science.280.5363.560.
Rytz, D. W., & Baiker, A. (1991). Partial oxidation of methane to methanol in a flow reactor at elevated pressure. Industrial & Engineering Chemistry Research, 30, 2287–2292. DOI: 10.1021/ie00058a007.
Takeguchi, M., & Okura, I. (1999). The role iron and copper in particulate methane monooxygenase of Methylosinus trichosporium OB3b. Journal of Molecular Catalysis A: Chemical, 137, 161–168. DOI: 10.1016/S1381-1169(98)00123-X.
Takeguchi, M., & Okura, I. (2000). Role of iron and copper in particulate methane monooxygenase of Methylosinus trichosporium OB3b. Catalysis Surveys from Japan, 4, 51–63. DOI: 10.1023/A:1019036105038.
Thomas, D. J., Willi, R., & Baiker, A. (1992). Partial oxidation of methane: the role of surface reactions. Industrial & Engineering Chemistry Research, 31, 2272–2278. DOI: 10.1021/ie00010a003.
Wilkinson, T. G., & Harrison, D. E. (1973). The affinity methane and methanol of mixed cultures grown on methane in continuous culture. Journal of Applied Bacteriology, 36, 309–313. DOI: 10.1111/j.1365-2672.1973.tb04107.x.
Wittenbury, R., Phillips, K. C., & Wilkinson, J. G. (1970). Enrichment isolation and some properties of methane utilizing bacteria. Journal of General Microbiology, 61, 205–218.
Xin, J. Y., Cui, J. R., Niu, J. Z., Hua, S. F., Xia, C. G., Li, S.B., & Li, M. Z. (2004). Production of methanol from methane by methanotrophic bacteria. Biocatalysis & Biotransformation, 22, 225–229. DOI: 10.1080/10242420412331283305.
Yamada, Y., Ueda, A., Shioyama, H., & Kobayashi, T. (2003). High throughput experiments on methane partial oxidation using molecular oxygen over silica doped with various elements. Applied Catalysis A: General, 254, 45–58. DOI: 10.1016/S0926-860X(03)00262-X.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markowska, A., Michalkiewicz, B. Biosynthesis of methanol from methane by Methylosinus trichosporium OB3b. Chem. Pap. 63, 105–110 (2009). https://doi.org/10.2478/s11696-008-0100-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-008-0100-5