Abstract
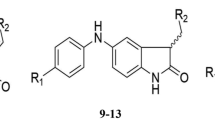
A QSAR study on a series of pyrimidinyl and triazinyl amines was performed to explore the physico-chemical parameters responsible for their anti-HIV activity and cytotoxicity. Physico-chemical parameters were calculated using WIN CAChe 6.1. Stepwise multiple linear regression analysis was carried out to derive QSAR models which were further evaluated for statistical significance and predictive power by internal and external validation. The selected best QSAR models showed correlation coefficient R of 0.914 and 0.901, and cross-validated squared correlation coefficient Q 2 of 0.685 and 0.691 for anti-HIV activity and cytotoxicity, respectively. The developed significant QSAR model indicates that hydrophobicity of the whole molecule plays an important role in the anti-HIV activity and cytotoxicity of pyrimidinyl and triazinyl amine derivatives. When hydrophobicity is increased, anti-HIV activity of the present series of compounds is decreased leading to high cytotoxicity.
Similar content being viewed by others
References
Barre-Sinoussi, F., Chermann, J. C., Rey, F., Nugeyre, M. T., Chamaret, S., Gruest, J., Dauguet, C., Axler-Blin, C., Vezinet-Brun, F., Rouzioux, C., Rozenbaum, W., & Montagnier, L. (1983). Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 220, 868–871. DOI: 10.1126/science.6189183.
Bhhatarai, B., & Garg, R. (2005). From SAR to comparative QSAR: role of hydrophobicity in the design of 4-hydroxy-5,6-dihydropyran-2-ones HIV-1 protease inhibitors, Bioorganic & Medicinal Chemistry, 13, 4078–4084. DOI: 10.1016/j.bmc.2005.03.049.
Buolamwini, J. K., & Assefa, H. (2002). CoMFA and CoM-SIA 3D QSAR and docking studies on conformationally-restrained cinnamoyl HIV-1 integrase inhibitors: Exploration of a binding mode at the active site. Journal of Medicinal Chemistry, 45, 841–852. DOI: 10.1021/jm010399h.
De Clercq, E. (1995). Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. Journal of Medicinal Chemistry, 38, 2491–2517. DOI: 10.1021/jm00014a001.
Gallo, R. C., Salahuddin, S. Z., Popovic, M., Shearer, G. M., Kaplan, M., Haynes, B. F., Palker, T. J., Redfield, R., Oleske, J., & Safai, B. (1984). Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science, 224, 500–503. DOI: 10.1126/science.6200936.
Garg, R., & Patel, D. (2005). Hydrophobicity in the design of P2/P2′ tetrahydropyrimidinone HIV protease inhibitors. Bioorganic & Medicinal Chemistry Letters, 15, 3767–3770. DOI: 10.1016/j.bmcl.2005.05.087.
Kumar, S., Jacob, R. R., & Tiwari, M. (2005). 3D-QSAR study of some 5,6-dihydropyran-2-ones as protease inhibitors. Indian Journal of Pharmaceutical Sciences, 67, 30–36.
Milton, J., Slater, M. J., Bird, A. J., Spinks, D., Scott, G., Price, C. E., Downing, S., Green, D. V. S., Madar, S., Bethell, R., & Stammers, D. K. (1998). Biaryl acids: Novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorganic & Medicinal Chemistry Letters, 8, 2623–2628. DOI: 10.1016/s0960-894X (98) 00214-5.
Nair, A. C., Jayatilleke, P., Wang, X., Miertus, S., & Welsh, W. J. (2002). Computational studies on tetrahydropyrimidine-2-one HIV-1 protease inhibitors: improving three-dimensional quantitative structure-activity relationship comparative molecular field analysis models by inclusion of calculated inhibitor-and receptor based properties. Journal of Medicinal Chemistry, 45, 973–983. DOI: 10.1021/jm010417v.
Pungpo, P., & Hannongbua, S. (2000). Three-dimensional quantitative structure-activity relationships study on HIV-1 reverse transcriptase inhibitors in the class of dipyridodiazepinone derivatives, using comparative molecular field analysis. Journal of Molecular Graphics and Modelling, 18, 581–590. DOI: 10.1016/S1093-3263 (00) 00053-X.
Raghavan, K., Buolamwini, J. K., Fesen, M. R., Pommier, Y., Kohn, K. W., & Weinstein, J. N. (1995). Three-dimensional quantitative structure-activity relationships (QSAR) of HIV integrase inhibitors: A comparative molecular field analysis (CoMFA) study. Journal of Medicinal Chemistry, 38, 890–897. DOI: 10.1021/jm00006a006.
Ravichandran, V., & Agrawal, R. K. (2007). Predicting anti-HIV activity of PETT derivatives: CoMFA approach. Bioorganic & Medicinal Chemistry Letters, 17, 2197–2202. DOI: 10.1016/j.bmcl.2007.01.103.
Ravichandran, V., Jain, P. K., Mourya, V. K., & Agrawal, R. K. (2007a). QSAR study on some arylsulfonamides as anti-HIV agents. Medicinal Chemistry Research, 16, 342–351. DOI: 10.1007/s00044-007-9034-7.
Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2007b). QSAR study of novel 1,1,3-trioxo [1,2,4]-thiadiazine (TTDs) analogues as potent anti-HIV agents. ARKIVOC, 2007(XIV), 204–212.
Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2008a). Prediction of HIV-1 protease inhibitory activity of 4-hydroxy-5,6-dihydropyran-2-ones: QSAR study. Journal of Enzyme Inhibition & Medicinal Chemistry (in press).
Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2008b). QSAR modeling of HIV-1 reverse transcriptase inhibitory activity with PETT derivatives. Digest Journal of Nanomaterials & Biostructures, 3, 9–17.
Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2008c). QSAR analysis of 6-aryl-2,4-dioxo-5-hexenoic acids as HIV-1 integrase inhibitors. Indian Journal of Pharmaceutical Education & Research, 42, 133–140.
Ravichandran, V., Prashanthakumar, B. R., Sankar, S., & Agrawal, R. K. (2008d). Comparative molecular similarity indices analysis for predicting anti-HIV activity of phenyl ethyl thiourea (PET) derivatives. Medicinal Chemistry Research, 17, 1–11. DOI: 10.1007/s00044-007-9087-7.
Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2008e). QSAR prediction of HIV-1 reverse transcriptase inhibitory activity of benzoxazinone derivatives. Internet Electronic Journal of Molecular Design (in press).
Sahu, K. K., Ravichandran, V., Jain, P. K., Sharma, S., Mourya, V. K., & Agrawal, R. K. (2008). QSAR analysis of chicoric acid derivatives as HIV-1 integrase inhibitors. Acta Chimica Slovenica, 55, 138–145.
Sahu, K. K., Ravichandran, V., Mourya, V. K., & Agrawal, R. K. (2007). QSAR analysis of caffeoyl naphthalene sulphonamide derivatives as HIV-1 Integrase inhibitors. Medicinal Chemistry Research, 15, 418–430. DOI: 10.1007/s00044-006-0020-2.
Thakur, V. V., Kim, J. T., Hamilton, A. D., Bailey, C. M., Domaoal, R. A., Wang, L., Anderson, K. S., & Jorgensen, W. L. (2006). Optimization of pyrimidinyl-and triazinyl-amines as non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorganic & Medicinal Chemistry Letters, 16, 5664–5667. DOI: 10.1016/j.bmcl.2006.08.037.
Tropsha, A., Gramatica, P., & Gombar, V. K. (2003). The importance of being earnest: Validation is the absolute essential for successful application and interpretation of QSPR models. QSAR & Combinatorial Science, 22, 69–77. DOI: 10.1002/qsar.200390007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravichandran, V., Jain, A., Mourya, V. et al. Prediction of anti-HIV activity and cytotoxicity of pyrimidinyl and triazinyl amines: A QSAR study. Chem. Pap. 62, 596–602 (2008). https://doi.org/10.2478/s11696-008-0072-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-008-0072-5