Abstract
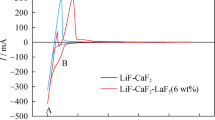
The electrochemical behaviour of lanthanum fluoride dissolved in molten lithium fluoride and in eutectic mixture LiF-CaF2 was investigated by cyclic voltammetry and laboratory electrolysis. The cyclic voltammetry experiments were carried out at 900°C and 800°C, respectively, in a graphite crucible (counter electrode). Several types of working electrodes (Mo, W, Ni and Cu) were used. Ni/Ni(II) was used as a reference electrode. Laboratory electrolysis was carried out in the system LiF-CaF2-LaF3 at 800°C in galvanostatic (j c = −0.21 A cm−2) and potentiostatic (E = 0.87 V) regimes. In both cases, nickel served as the cathode and graphite as the anode. It was found that no new separate reduction peak occurred on the molybdenum or tungsten electrodes in the investigated systems. When copper or nickel electrodes were used, new peaks corresponding to the reduction of lanthanum(III) to lanthanum metal appeared. This can be explained by the formation of alloys or intermetallic compounds of lanthanum with copper or nickel. X-ray microanalysis showed that lanthanum was electrodeposited together with calcium under formation of intermetallic compounds with the electrode materials in the galvanostatic regime. In the potentiostatic regime, mainly lanthanum was deposited, which enabled its separation.
Similar content being viewed by others
References
Borzone, G., Parodi, N., Raggio, R., & Ferro, R. (2004). Thermodynamic investigation of samarium-nickel alloys. Journal of Alloys and Compounds, 317–318, 532–536. DOI: 10.1016/S0925-8388(00)01382-7.
Castrillejo, Y., Bermejo, M. R., Barrado, E., Martínez, A. M., & Díaz Arocas, P. (2003). Solubilization of rare earth oxides in the eutectic LiCl-KCl mixture at 450°C and in the equimolar CaCl2-NaCl melt at 550°C. Journal of Electro-analytical Chemistry, 545, 141–157. DOI: 10.1016/S0022-0728(03)00092-5.
Chamelot, P., Massot, L., Hamel, C., Nourry, C., & Taxil, P. (2007). Feasibility of the electrochemical way in molten fluorides for separating thorium and lanthanides and extracting lanthanides from the solvent. Journal of Nuclear Materials, 360, 64–74. DOI: 10.1016/j.jnucmat.2006.08.015.
Chase M. W. (1998). NIST-JANAF, Thermo chemical tables (4th ed.). Journal of Physics and Chemical Reference Data, Mononograph No. 9. Malville, NY: NIST.
Cordoba, G., & Caravaca, C. (2004). An electrochemical study of samarium ions in the molten eutectic LiCl + KCl. Journal of Electroanalytical Chemistry, 572, 145–151. DOI: 10.1016/j.jelechem.2004.05.029.
Greef, R., Peat, R., Peter, L. M., Pletcher, D., & Robinson, J. (2001). Instrumental methods in electrochemistry. Chichester: Ellis Horwood Ltd; New York: John Wiley & Sons Inc.
Hamel, C., Chamelot, P., & Taxil, P. (2004). Neodymium(III) cathodic processes in molten fluorides. Electrochimica Acta, 49, 4467–4476. DOI: 10.1016/j.electacta.2004.05.003.
Iida, T., Nohira, T., & Ito, Y. (2001). Electrochemical formation of Sm-Ni alloy films in a molten LiCl-KCl-SmCl3 system. Electrochimica Acta, 46, 2537–2544. DOI: 10.1016/S0013-4686(01)00470-4.
Iida, T., Nohira, T., & Ito, Y. (2003). Electrochemical formation of Sm-Co alloy films by Li codeposition method in a molten LiCl-KCl-SmCl3 system. Electrochimica Acta, 48, 901–906. DOI: 10.1016/S0013-4686(02)00786-7.
Massalski, T., & Okamoto, H. (Eds.) (1990). Binary alloy phase diagrams (2nd ed.). Materials Park, OH: ASM International.
Massot, L., Chamelot, P., & Taxil, P. (2005). Cathodic behaviour of samarium(III) in LiF-CaF2 media on molybdenum and nickel electrodes. Electrochimica Acta, 50, 5510–5517. DOI: 10.1016/j.electacta.2005.03.046.
Mottot, Y. (1986). Thesis, University of Paris VI, Paris.
Souček, P., Chuchvalcová-Bímová, K., Lisý, F., & Tuláčková, R. (2005). Study of electrochemical separation methods for application within MSR fuel cycle. In Proceedings of 7th International Symposium on Molten Salts Chemistry & Technology, August 29–September 2, 2005 (Vol. II, pp. 701–704). Toulouse: University Paul-Sabatier of Toulouse III.
Stefanidaki, E., Hasiotis, C., & Kontoyannis, C. (2001). Electrodeposition of neodymium from LiF-NdF3-Nd2O3 melts. Electrochimica Acta, 46, 2665–2670. DOI: 10.1016/S0013-4686(01)00489-3.
Su, X., Zhang, W., & Du, Z. (1998). A thermodynamic assessment of the Ni-Sm system. Journal of Alloys and Compounds, 278, 182–184. DOI: 10.1016/S0925-8388(98)00560-X.
Uhlíř, J. (2007). Advanced fuel cycles and Molten-Salt Reactors. In AER Working Group “f”, Spent Fuel Transmutations, April 10–13, 2007. Třešt’, Czech Republic. (http://www.trest2005.vjv.cz/).
Zvejskova, R., Lisy, F., & Soucek, P. (2002). Development of electrochemical separations of uranium and RE elements from fluoride melts. In Seventh Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation, October 14–16, 2002. Jeju (Republic of Korea): Nuclear Energy Agency. (http://www.nea.fr/html/pt/docs/iem/jeju02/session2/SessionII-09.pdf).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambrová, M., Jurišová, J. & Danielik, V. Electrochemical behaviour of lanthanum fluoride in molten fluorides. Chem. Pap. 62, 559–565 (2008). https://doi.org/10.2478/s11696-008-0068-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-008-0068-1