Abstract
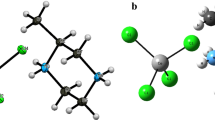
Correlations involving bond lengths and bond angles in the molecular structure of the Cu4OCl6(4-Mepy)4 complex (4-Mepy = 4-methylpyridine) with four symmetrically independent molecules present in the unit cell showed that the donor-acceptor behavior involving the π-back donation into the pyridine rings of the 4-Mepy ligands is most effectively stimulated by a suitable orientation of the pyridine rings in the trigonal bipyramidal geometry. The pyridine ring planes are almost in parallel orientation with one of the three Cu-Cl bonds. The bond lengths of these Cu-Cl bonds are in a significant linear correlation with the Cu-N bond lengths and the bonds lengths of the pyridine rings. The pyridine rings orientation is affected by distortion of the trigonal bipyramidal geometry to tetragonal pyramidal coordination, by out-of plane pyridine rings deviation and in-plane pyridine rings tilting, by puckering of the pyridine rings and by the effects of the methyl groups. The pyridine rings in at least seven of the sixteen trigonal bipyramidal coordinations exhibit an orientation supporting the π-back bonding between the Cu(II) atoms and the pyridine rings.
Similar content being viewed by others
References
Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., & Verschoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. Journal of Chemical Society, Dalton Transactions, 1984, 1349–1356. DOI: 10.1039/DT9840001349.
Anson, C. E., arapKoske, S. K., Jayasooriya, U. A., & Cannon, R. D. (1992). FT-IR spectroscopy: a sensitive probe of concealed symmetry-lowering in tetranuclear copper(II) complexes. Spectrochimica Acta Part A: Molecular Spectroscopy, 48A, 151–154. DOI: 10.1016/0584-8539(92)80017-Q.
Berkesi, O., Andor J. A., Jayasooriya, U. A., & Cannon, R. D. (1992). Vibrations of central oxygen in a high-symmetry tetranuclear metal cluster: a definitive assignment using isotopic substitution in a basic zinc carboxylate complex. Spectrochimica ActaPart A: Molecular Spectroscopy, 48A, 147–149. DOI: 10.1016/0584-8539(92)80016-P.
Gill, N. S., & Sterns, M. (1970). The preparation and properties of μ 4-oxo-hexa-μ-chloro-tetrakis[(2-methylpyridine)copper (II)] hydrate, Cu4OCl6(2-mepy)4 · xH2O, and di-μ-methoxo-bis[chloro(2-methylpyridine)copper(II)], [CuCl(OCH3)(2-mepy)]2, and X-ray structure analysis of Cu4OCl6(2-mepy)4 · xH2O. Inorganic Chemistry, 9, 1619–1625. DOI: 10.1021/ic50089a004.
Hendl, J. (2006). Přehled statistických metod zpracování dat. Analýza a metanalýza dat (2nd ed.) (p. 246). Praha: Portál.
Huheey, J. E. (1983). Inorganic chemistry. Principles of structure and reactivity (p. 440). New York: Harper and Row.
Jorík, V., Koman, M., Makáňová, D., Mikloš, D., Broškovičová, A., & Ondrejovič, G. (1996). Fine stereochemical effects in tetranuclear mixed-halide copper(II) complexes: The structure of Cu4OBr3Cl3(OPPh3)4. Polyhedron, 15, 3129–3137. DOI: 10.1016/0277-5387(96)00013-7.
Kilbourn, B. T., & Dunitz, J. D. (1967). The crystal and molecular structure of μ 4-oxohexa-μ-chlorotetrakis(pyridine copper(II)), Cu4Cl6O.4C5H5N, a polynuclear copper complex. Inorganica Chimica Acta, 1, 209–216. DOI: 10.1016/S0020-1693(00)93172-4.
Londergan, C. H., & Kubiak, C. P. (2003). Vibronic participation of the bridging ligand in electron transfer and delocalization: New application of a three-state model in pyrazine-bridged mixed-valence complexes of trinuclear ruthenium clusters. Journal of Physical Chemistry A, 107, 9301–9311. DOI: 10.1021/jp035643q.
Murphy, B., & Hathaway, B. (2003). The stereochemistry of the copper(II) ion in the solid state — some recent perspectives linking the Jahn-Teller effect, vibronic coupling, structure correlation analysis, structural pathways and comparative X-ray crystallography. Coordination Chemistry Reviews, 243, 237–262. DOI: 10.1016/80010-8545(03)00084-5.
Murphy, G., O’sullivan, C., Murphy, B., & Hathaway, B. (1998). Comparative crystallography. 5. Crystal structures, electronic properties, and structural pathways of five [Cu(phen)2 Br][Y] complexes, Y = [Br]−·H2O, [ClO4]−, [NO3]− H2O, [PF6]−, and [BPh4]−. Inorganic Chemistry, 37, 240–248. DOI: 10.1021/ic970458a.
Norman, R. E., Rose, N. J., & Stenkamp, R. E. (1989). Simple, direct synthesis and structure of hexa-μ-chloro-tetrakis(1-methylimidazole)-μ 4-oxo-tetracopper(II). Acta Crystallographica, C45, 1707–1713. DOI: 0108-2701/89/111707.
Ondrejovič, G., Koman, M., & Kotočová, A. (2008). Structural and electronic effects involving pyridine rings in 4-methylpyridine Cu4OX6L4 complexes. I. Vibrational spectra of Cu4OBrnCl(6−n)(4-Mepy)4 complexes. Chemical Papers, 62, 480–486. DOI: 10.2478/s11696-008-0055-6.
Ondrejovič, G., & Kotočová, A. (2006). Spectral and electrochemical study of coordination molecules Cu4OX6L4: 3-Methylpyridine and 4-methylpyridine Cu4OBrnCl(6−n)L4 complexes. Chemical Papers, 60, 10–21. DOI: 10.2478/s11696-006-0003-2.
tom Dieck, H. (1973). Tetranuclear complexes of trigonalbipyramidal copper(II). III. Electronic and infrared spectra. Inorganica Chimica Acta, 7, 397–403. DOI: 10.1016/S0020-1693(00)94852-7.
Triola, M. F. (1989). Elementary statistics (4th ed.). Redwood City, CA: Benjamin-Cummings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ondrejovič, G., Koman, M. & Kotočová, A. Structural and electronic effects involving pyridine rings in 4-methylpyridine Cu4OX6L4 complexes. II. Correlations based on molecular structure of the Cu4OCl6(4-Mepy)4 complex. Chem. Pap. 62, 566–574 (2008). https://doi.org/10.2478/s11696-008-0067-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-008-0067-2