Abstract
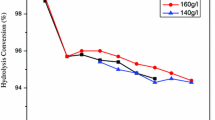
The influence of TiOSO4 and free sulphuric acid concentrations in the starting solution on the degree of titanyl sulphate conversion to hydrated titanium dioxide and post-hydrolytic sulphuric acid was studied. Titanyl sulphate solution, an intermediate product in the commercial preparation of titanium dioxide pigments by sulphate route, was used. It was found that the degree of hydrolysis markedly depends on the studied parameters. The lower was the content of TiOSO4 in the starting solution, the higher conversion was achieved. The degree of hydrolysis at the final stage varied between 81 % (420 g TiOSO4 dm−3, 216 g H2SO4 dm−3) and 92 % (300 g TiOSO4 dm−3, 216 g H2SO4 dm−3). The same relation was obtained when changing the concentration of free H2SO4 in the starting solution. The degree of hydrolysis at the final stage varied between 49 % (261 g H2SO4 dm−3, 340 g TiOSO4 dm−3) and 96 % (136 g H2SO4 dm−3, 340 g TiOSO4 dm−3). The particle size of the obtained hydrated titanium dioxide (HTD) also depends on the initial solution composition.
Similar content being viewed by others
References
Bavykin, D. V., Dubovitskaya, V. P., Vorontsov, A. V., & Parmon, V. N. (2007). Effect of TiOSO4 hydrotermal hydrolysis conditions on TiO2 morphology and gas-phase oxidative activity. Research on Chemical Intermediates, 33, 449–464. DOI: 10.1163/156856707779238702.
Bavykin, D. V., Savinov, E. N., & Smirniotis, P. G. (2003). Kinetics of the TiO2 films growth at the hydrothermal hydrolysis of TiOSO4. Reaction Kinetics and Catalysis Letters, 79, 77–84. DOI: 10.1023/A:1024107701071.
Blumenfeld, J. (1924). U.S. Patent No.1,504,672. Washington, D.C.: U.S. Patent Office.
Cheng, H., Ma, J., Zhao, Z., & Qi, L. (1995). Hydrotermal preparation of uniform nanosize rutile and anatase particles. Chemistry of Materials, 7, 663–671. DOI: 10.1021/cm00052a010.
Chung, F. H., & Smith, D. K. (2000). Industrial application of X-ray diffraction. New York-Basel: Marcel Dekker, Inc.
Dąbrowski, W., Tymejczyk, A., & Lubkowska, A. (2001). Włlaściwości i zastosowanie pigmentów dwutlenku tytanu (Properties and application of titanium dioxide pigments). Police: Chemical Works “Police” S.A.
Dobrovolskii, I. P. (1988). Khimia i tekhnologia oksidnyh sojedinenii titana (The chemistry and technology of the oxide compounds of titanium). Sverdlovsk: UrO AN SSSR.
Grzmil, B., Grela, D., & Kic, B. (2006). Studies on the hydrolysis process of titanium sulfate compounds. Polish Journal of Chemical Technology, 8(3), 19–21.
Gussman, N. (2005). Titanium dioxide: from black sand to white pigment. Chemical Engineering Progress, 101(6), 64–64.
Hidalgo, M. C., & Bahnemann, D. (2005). Highly photoactive supported TiO2 prepared by thermal hydrolysis of TiOSO4: optimisation of the method and comparison with other synthetic routes. Applied Catalysis B: Enviromental, 61, 259–266. DOI: 101016/j.apeatb.2005.06.004.
Karvinen, S. (1995). U.S. Patent No. 5,443,811. Washington, D.C.: U.S. Patent and Trademark Office.
Lawes, G., & Jame, A. M. (1987). Scanning electron microscopy and X-ray microanalysis. Chichester: John Wiley & Sons Ltd.
Mackey, T. S. (1974). Acid leaching of ilmenite into synthetic rutile. Industrial & Engineering Chemistry Product Research and Development, 13, 9–17. DOI: 10.1021/i360049a003.
Marczenko, Z. (1979). Spectrofotometryczne oznaczanie pierwiastkow (Spectrophotometric determination of elements). Warsaw: PWN.
Mecklenburg, W. (1930). U.S. Patent No. 1,758,528. Washington, D.C.: U.S. Patent Office.
Perera, S., Zelenski, N., & Gillan, G. (2006). Synthesis of nanocrystalline TiO2 and reduced titanium oxides via rapid and exothermic metathesis reactions. Chemistry of Materials, 18, 2381–2388. DOI: 10.121/cm0528328.
Przepiera, A., & Sosnowski, J. (1998). Możliwości udoskonalenia siarczanowej technologii produkcji ditlenku tytanu. Przemysł Chemiczny, 77, 328–334.
Skudlarski, K. (1974). Technologia produkcji tytanu i dwutlenku tytanu. Wrocław: Politechnika Wrocławska.
Ullmann’s Encyclopedia of Industrial Chemistry. (2002). Weinheim: Wiley-VCH Verlag GmbH.
Wiederhöft, G., Bayer, E., Müller, W. D., & Lailach, G. (1991). U.S. Patent No. 4,988,495. Washington, D.C.: U.S. Patent and Trademark Office.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grzmil, B.U., Grela, D. & Kic, B. Hydrolysis of titanium sulphate compounds. Chem. Pap. 62, 18–25 (2008). https://doi.org/10.2478/s11696-007-0074-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/s11696-007-0074-8