Abstract
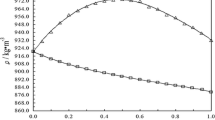
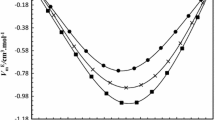
The densities of the (cyclohexane + pentane, or hexane, or heptane, or octane, or nonane) systems were measured at the temperature 298.15 K by means of a vibrating-tube densimeter. Their respective excess molar volumes were calculated and correlated using the fourth-order Redlich—Kister equation, with the maximum likelihood principle being applied in the determination of the adjustable parameters. The values of excess molar volumes were negative for the cyclohexane + pentane system, whereas they were positive for the other systems with the values increasing with the number of carbon atoms in the respective alkane molecules.
Similar content being viewed by others
References
Riddick, A., Bunger, W. B., and Sakano, T. K., Organic Solvents, Physical Properties and Methods of Purification, Vol. II, 4th Edition. Willey, New York, 1986.
Takenaka, M., Tanaka, R., and Murakami, S., J. Chem. Thermodyn. 12, 849 (1980).
Awwad, A. M. and Salman, M. A., Fluid Phase Equilib. 25, 195 (1986).
Iloukhani, H. and Rezaei-Sameti, M., J. Chem. Eng. Data 50, 1928 (2005).
Goates, J. R., Ott, J. B., and Grigg, R. B., J. Chem. Thermodyn. 11, 497 (1979).
Letcher, T. M. and Spiteri, W. L., J. Chem. Thermodyn. 11, 435 (1979).
Aminabhavi, T. M., Patil, V. B., Aralaguppi, M. I., and Phayde, H. T. S., J. Chem. Eng. Data 41, 521 (1996)
Sanchez-Pajares, R. G. and Nuñez Delgado, J., J. Chem. Thermodyn. 11, 815 (1979).
Pandey, J. D., Jain, P., and Vyas, V., PRAMANA — J. Phys. 43, 361 (1994).
Vyas, V. and Nautiyal, T., PRAMANA — J. Phys. 59, 663 (2002).
Tripathi, N., Int. J. Thermophys. 26, 693 (2005).
Mascato, E., Mosteiro, L., Piñeiro, M. M., García, J., Iglesias, T. P., and Legido, J. L., J. Chem. Thermodyn. 33, 269 (2001).
Zheng, G. K., Schmitter, B. M., Knobler, C. M., and Scott, R. L., J. Chem. Thermodyn. 16, 943 (1984).
Ott, J. B., Marsh, K. N., and Stokes, R. H., J. Chem. Thermodyn. 12, 1139 (1980).
Goates, J. R., Ott, J. B., and Moellmer, J. F., J. Chem. Thermodyn. 9, 249 (1977).
Takigawa, T., Ohba, M., Ogawa, H., and Murakami, S., Fluid Phase Equilib. 204, 119 (2003).
Letcher, T. M., J. Chem. Thermodyn. 7, 205 (1975).
Romani, L. and Paz Andrade, M. I., An. Quim. 71, 3 (1975).
Gómez-Ibáñez, J. and Liu, C. T., J. Phys. Chem. 65, 2148 (1961).
Fenby, D. V., Khurma, J. R., Kooner, Z. S., Block, T. E., Knobler, C. M., Reeder, J., and Scott, R. L., Aust. J. Chem. 33, 1927 (1980).
Iloukhani, H., Rezaei-Sameti, M., and Zarei, H. A., Thermochim. Acta 438, 9 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balán, J., Morávková, L. & Linek, J. Excess molar volumes of the (cyclohexane + pentane, or hexane, or heptane, or octane, or nonane) systems at the temperature 298.15 K. Chem. Pap. 61, 497–501 (2007). https://doi.org/10.2478/s11696-007-0068-6
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/s11696-007-0068-6