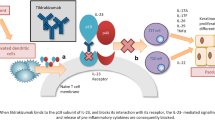
Abstract
Efalizumab is a recombinant humanized monoclonal antibody approved for the treatment of adult patients with moderate-to-severe chronic plaque psoriasis. We present six cases of favorable response to efalizumab therapy in patients with psoriasis who demonstrated inadequate response or who were nonresponders to treatment with etanercept, a tumor necrosis factor-α-binding fusion protein. The subsequent response of these patients suggests that efalizumab may be a viable treatment option for patients with psoriasis who respond poorly to etanercept.




Similar content being viewed by others
References
Jullien D, Prinz JC, Langley RGB, et al. T-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva™): mechanisms of action. Dermatology 2004; 208: 297–306
Krueger GG. Current concepts and review of alefacept in the treatment of psoriasis. Dermatol Clin 2004; 22 (4): 407–26, viii
Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152 (6): 1304–12
Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, doubleblind trial. Lancet 2005; 366 (9494): 1367–74
Gottlieb AB, Feldman S, Weinstein G, et al. Infliximab: one year results [poster no. P43]. 64th Annual Meeting of the American Academy of Dermatology; 2006 Mar 3-7; San Francisco (CA)
Langley R, Leonardi C, Toth D, et al. Long-term safety and efficacy of adalimumab in the treatment of moderate to severe chronic plaque psoriasis [abstract]. 14th European Academy of Dermatology and Venereology Congress; 2005 Oct 12-15; London
Tyring S, Poulin Y, Langley R, et al. A 96-week phase III study of safety and efficacy of etanercept 50mg twice weekly in patients with psoriasis [abstract]. J Am Acad Dermatol 2006; 54 Suppl. 3: AB10
Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003; 290 (23): 3073–80
Menter A, Hamilton TK, Caro I, et al. Safety and tolerability of efalizumab therapy for patients with moderate to severe chronic plaque psoriasis: results of an open-label phase IIIb multicenter trial [abstract]. 63rd Annual Meeting of the American Academy of Dermatology; 2005 Feb 18-22; New Orleans (LA)
Gottlieb AB, Gordon KB, Hamilton TK, et al. Maintenance of efficacy and safety with continuous efalizumab therapy in patients with moderate to severe chronic plaque psoriasis: final phase IIIb study results [abstract]. 63rd Annual Meeting of the American Academy of Dermatology; 2005 Feb 18-22; New Orleans (LA)
Krueger GG, Feldman SR, Camisa C, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis? J Am Acad Dermatol 2000; 43 (2 Pt 1): 281–5
Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol 2005; 152 (5): 861–7
Enbrel® (etanercept) [package insert]. Thousand Oaks (CA): Immunex Corporation, 2006 Apr
Raptiva® (efalizumab) [package insert]. South San Francisco (CA): Genentech, Inc., 2005 Jun
Acknowledgments
The authors received no direct compensation for preparation of this manuscript. Patricia Segarini, PhD, Sabrina Cheng, MD, and Blesila Castro, PhD, of Helix Medical Communications LLC provided medical writing support on behalf of Genentech, Inc. Dr Kramer acts as a consultant to and has received speaker’s honoraria from Amgen, Genentech, and Abbott. Dr Turner is as an advisor, principal investigator, and member of the speakers’ bureau for Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kramer, J.M., Turner, J.E. Efalizumab Therapy for Psoriasis in Patients with Inadequate Responses to Etanercept. Am J Clin Dermatol 10, 134–140 (2009). https://doi.org/10.2165/00128071-200910020-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200910020-00008