Abstract
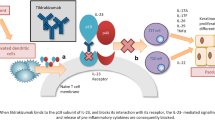
▴ Efalizumab is a humanized monoclonal antibody that binds to CD11a, the α-subunit of lymphocyte function-associated antigen-1, and consequently inhibits T-cell activation.
▴ In randomized, double-blind, placebo-controlled trials, efalizumab 1.0 mg/kg, administered subcutaneously once weekly for 12 weeks, significantly reduced disease activity in patients with chronic, moderate-to-severe plaque psoriasis. Significantly more efalizumab recipients had a ≥75% decrease in the Psoriasis Area and Severity Index (PASI) score [22.4–38.9%] than placebo recipients (2.4–4.9%); an additional 12 weeks of treatment resulted in sustained or increased PASI responses.
▴ The efficacy of weekly subcutaneous efalizumab was maintained during 15 months of treatment.
▴ Efalizumab significantly improved health-related quality of life in patients with chronic plaque psoriasis, with significant improvements in all the Dermatology Life Quality Index domains.
▴ Efalizumab was generally well tolerated in patients with chronic, moderate-to-severe plaque psoriasis, with few serious adverse events or treatment withdrawals. The most common adverse events were headache, chills, myalgia, pain, and fever; these most often occurred within 2 days of administration of the drug, were most frequent after the first or second dose, and decreased in frequency over time.



Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Cather JC, Cattier JC, Menter A. Modulating T cell responses for the treatment of psoriasis: a focus on efalizumab. Expert Opin Biol Ther. 2003 Apr; 3 (2): 361–70
Lebwohl M. Psoriasis. Lancet. 2003; 361: 1197–204
Pariser DM. Management of moderate to severe plaque psoriasis with biologic therapy. Manag Care. 2003 Apr; 12 (4): 36–44
Agarwal R, Vij N, Katare OP. Biotherapy: a novel approach in the treatment of psoriasis. Drugs Future. 2002; 27 (11): 1071–7
Sobell JM, Hallas SJ. Systemic therapies for psoriasis: understanding current and newly emerging therapies. Semin Cutan Med Surg. 2003; 22 (3): 187–95
Leonardi CL. Efalizumab: an overview. J Am Acad Dermatol. 2003 Aug; 49 (2 Suppl.): S98–104
Prinz JC. The role of T cells in psoriasis. J Fur Acad Dermatol Venereol. 2003; 17 (3): 257–70
Schön MP, Zollner TM, Boehncke WH. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J Invest Dermatol. 2003; 121 (5): 951–62
Bauer RJ, Dedrick RL, White ML, et al. Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm. 1999 Aug; 27 (4): 397–420
Gottlieb AB, Krueger JG, Wittkowski K, et al. Psoriasis as a model for T-cell-mediated disease: immunobiologic and clinical effects of treatment with multiple doses of efalizumab, an anti-CD1la antibody. Arch Dermatol. 2002 May; 138 (5): 591–600
Werther WA, Gonzalez TA, O’Connonr SJ, et al. Humanization of an antilymphocyte function-associated antigen (LFA)-1 monoclonal antibody and reengineering of the humanized antibody for binding rhesus LFA-1. J Immunol. 1996; 157 (11): 4986–95
Dedrick RL, Bauer R, Bohmann D, et al. Pharmacokinetics and pharmacodynamics of subcutaneously administered anti-CD11a (hu1124) in psoriasis subjects [abstract no. 505 plus poster]. 59th Annual Meeting American Academy of Dermatology; 2001 Mar 2–7; Washington (DC)
Gottlieb A, Krueger JG, Bright R, et al. Effects of administrations of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasis. J Am Acad Dermatol. 2000 Mar; 42: 428–35
Krueger J, Gottlieb A, Miller B, et al. Anti-CD11a treatment for psoriasis concurrently increases circulating T-cells and decreases plaque T-cells, consistent with inhibition of cutaneous T-cell trafficking [abstract no. HB9]. J Invest Dermatol. 2000 Aug; 115 (2): 333
Genetech Inc. Raptiv™ (efalizumab): for injection, subcutaneous [online]. Available from URL: http://www.gene.com/gene/products/information/pdf/raptiva-prescribing.pdf [Accessed 2005 Feb 25]
Leonardi CL, Papp KA, Gordon KB, et al. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol. 2005; 52 (3): 425–33
Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003 Dec 17; 290 (23): 3073–80
Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003 Nov 20; 349 (21): 2004–13
Gottlieb AB, Gordon KB, Lebwohl MG, et al. Extended efalizumab therapy sustains efficacy without increasing toxicity in patients with moderate to severe chronic plaque psoriasis. J Drugs Dermatol. 2004 Nov; 3 (6): 614–24
Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999; 141: 185–91
Menter A, Gordon K, Carey W, et al. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol. 2005 Jan; 141 (1): 31–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wellington, K., Perry, C.M. Efalizumab. Am J Clin Dermatol 6, 113–118 (2005). https://doi.org/10.2165/00128071-200506020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200506020-00006