Abstract
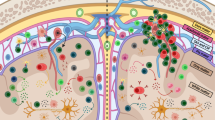
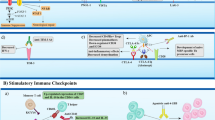
T-cell vaccination (TCV) is a unique approach to induce immune regulation that may have importance in the treatment of autoimmune diseases, including multiple sclerosis (MS). TCV employs a classic vaccine strategy of injecting an attenuated form of the disease-causing agent — in this case, myelin-reactive T cells — that have been selected and expanded from each MS donor and then re-injected after irradiation to induce protective immunity. This anti-T-cell immunity consistently results in selective deletion or regulation of the targeted pathogenic T cells in vivo. Longitudinal studies have established that TCV is safe and often results in a reduced relapse rate and clinical stability or improvement, at least temporarily, in the majority of treated MS patients. These results lend direct support to the involvement of inflammatory myelin-reactive T cells in the MS disease process. However, these hopeful trends reported in a number of pilot trials await validation in larger proof-of-principle trials that are now in progress.

Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
|References
Raine CS, Scheinberg LC. On the immunopathology of plaque development and repair in multiple sclerosis. J Neuroimmunol 1988; 20: 189–94
Charcot J-M. Histologie de la sclerose en plaques. Gazette des Hopitaux, Paris 1868; 41: 554–5
Trapp BD, Peterson J, Ransohoff RM. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–85
Steinman L. Multiple sclerosis: a two stage disease. Nat Immunol 2001; 2: 762–5
Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995; 80(5): 695–705
Hemmer BM, Vergelli B, Gran N, et al. Cutting edge: predictable TCR antigen recognition based on peptide scans leads to the identification of agonist ligands with no sequence homology. J Immunol 1998; 160: 3631–6
Vandenbark AA. TCR peptide vaccination in multiple sclerosis: boosting a deficient regulatory network that may involve TCR-specific CD4+CD25+ Treg cells. Curr Drug Targets Inflamm Allergy 2005; 4: 85–94
Lucchinetti C, Brack W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000; 47: 707–17
Wang C, Gold BG, Kaler LJ, et al. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem 2006; 98: 1817–27
Hellings N, Raus J, Stinissen P. Insights into the immunopathogenesis of multiple sclerosis. Immunol Res 2002; 25: 27–51
Olsson T, Wang WZ, Hojeberg B, et al. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J Clin Invest 1990; 86: 981–5
Sun JB, Olsson T, Wang WZ, et al. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol 1991; 21: 1461–8
Chou YK, Bourdette DN, Offner H, et al. Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J Neuroimmunol 1992; 38: 105–14
Ota K, Matsui M, Milford EL, et al. T cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 1990; 346: 183–5
Trotter JL, Pelfrey CM, Trotter AL, et al. T cell recognition of myelin proteolipid protein and myelin proteolipid protein peptides in the peripheral blood of multiple sclerosis and control subjects. J Neuroimmunol 1998; 84: 172–8
van Noort JM, vanSechel AC, Bajramovic JJ, et al. The small heat-shock protein a-B-crystallin as candidate autoantigen in multiple sclerosis. Nature 1995; 375: 798–801
deRosbo NK, Milo R, Lees MB, et al. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest 1993; 92: 2602–8
Bieganowska KD, Ausubel LJ, Modabber Y, et al. Direct ex vivo analysis of activated fas-sensitive autoreactive T cells in human autoimmune disease. J Exp Med 1997; 185: 1585–94
Zhang J, Markovic-Plese S, Lacet B, et al. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med 1994; 179: 973–84
Wilson DB, Golding AB, Smith RA, et al. Results of a phase I clinical trial of a T-cell receptor peptide vaccine in patients with multiple sclerosis: I, analysis of T-cell receptor utilization in CSF cell populations. J Neuroimmunol 1997; 76: 15–28
Allegretta M, Nicklas JA, Sriram S, et al. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science 1990; 247: 718–21
Lodge PA, Johnson C, Sriram S. Frequency of MBP and MBP peptide-reactive T cells in the HPRT mutant T-cell population of MS patients. Neurology 1996; 46: 1410–5
Trotter JL, Damico CA, Cross AH, et al. HPRT mutant T-cell lines from multiple sclerosis patients recognize myelin proteolipid protein peptides. J Neuroimmunol 1997; 75: 95–103
Vandenbark AA, Bourdette DN, Whitham R, et al. Episodic changes in T cell frequencies to myelin basic protein in patients with multiple sclerosis. Neurology 1993; 43: 2416–7
Vandenbark AA, Chou YK, Whitham R, et al. Treatment of multiple sclerosis with T cell receptor peptides: results of a double-blind pilot trial. Nature Med 1996; 2: 1109–15
Weiner HL, Mackin GA, Matsui M, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 1993; 259: 1321–4
Zhang J, Medaer R, Stinissen P, et al. MHC restricted clonotypic depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science 1993; 261: 1451–4
Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nature Med 2000; 6: 1167–75
Hemmer B, Stefanova I, Vergelli M, et al. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol 1998; 160: 5807–14
Ho HZ, Tiwari JL, Haile RW, et al. HLA-linked and unlinked determinants of multiple sclerosis. Immunogenetics 1982; 15: 509–17
Francis DA, Batchelor JR, McDonald WI, et al. Multiple sclerosis and HLA-DQw1. Lancet 1986; 1: 211
Kellar-Wood HF, Wood NW, Holmans P, et al. Multiple sclerosis and the HLA-D region: linkage and association studies. J Neuroimmunol 1995; 58: 183–90
Hauser SL. T-cell receptor genes: germline polymorphisms and genetic susceptibility to demyelinating diseases. Ann N Y Acad Sci 1995; 756: 233–40
Wucherpfennig KW, Sette A, Southwood S, et al. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med 1994; 179: 279–90
Noseworthy JH, Gold R, Hartung HP. Treatment of multiple sclerosis: recent trials and future perspectives. Curr Opin Neurol 1999; 12: 279–93
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910
Rudick RA, Stuart WH, Calabrese PA, et al. Natalizumab plus Interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354: 911–23
Bar-Or A. Effects of long-term fingolimod therapy on circulating mononuclear cell populations in multiple sclerosis [abstract]. Clin Immunol 2007; 123 Suppl.: 563
Muir M, Lovett-Racke AE, Racke MK. Novel therapeutic strategies targeting the pathogenic T cells in multiple sclerosis. Exp Rev Clin Immunol 2005; 1: 345–55
Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature 1981; 292: 60–1
Quintana FJ, Cohen IR. Anti-ergotypic immunoregulation. Scand J Immunol 2006; 64: 205–10
Vandenbark AA, Hashim G, Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature 1989; 341: 541–4
Howell MD, Winters ST, Olee T, et al. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science 1989; 246: 668–70
Zang YCQ, Hong J, Rivera VM, et al. Preferential recognition of TCR hypervariable regions by human anti-idiotypic T cells induced by T cell vaccination. J Immunol 2000; 164: 4011–7
Hong J, Zang YCQ, Nie H, et al. CD4+ regulatory T cell responses induced by T cell vaccination in patients with multiple sclerosis. Proc Natl Acad Sci U S A 2006; 103: 5024–9
Hellings N, Raus J, Stinissen P. T-cell-based immunotherapy in multiple sclerosis: induction of regulatory immune networks by T-cell vaccination. Expert Rev Clin Immunol 2006; 2: 705–16
van der Aa A, Hellings N, Medaer R, et al. T cell vaccination in multiple sclerosis patients with autologous CSF-derived activated T cells: results from a pilot study. Clin Exp Immunol 2003; 131: 155–68
Hafler DA, Cohen I, Benjamin DS, et al. T cell vaccination in multiple sclerosis: a preliminary report. Clin Immunol Immunopathol 1992; 62: 307–13
Medaer R, Stinissen P, Trayen L, et al. Depletion of myelin-basic-protein autoreactive T cells by T cell vaccination: pilot trial in multiple sclerosis. Lancet 1995; 346: 807–8
Hermans G, Denzer U, Lohse A, et al. Cellular and humoral immune responses against autoreactive T cells in multiple sclerosis patients after T cell vaccination. J Autoimmunity 1999; 13: 233–46
Zhang JZ, Rivera VM, Tejada-Simon MV, et al. T cell vaccination in multiple sclerosis: results of a preliminary study. J Neurol 2002; 249: 212–8
Correale J, Lund B, McMillan M, et al. T cell vaccination in secondary progressive multiple sclerosis. J Neuroimmunol 2000; 107: 130–9
Achiron A, Lavie G, Kishner I, et al. T cell vaccination in multiple sclerosis relapsing-remitting nonresponder patients. Clin Immunol 2004; 113: 155–60
Stinissen P, Zhang J, Medaer R, et al. Vaccination with autoreactive T cell clones in multiple sclerosis: overview of immunological and clinical data. J Neurosci Res 1996; 45: 500–11
Hermans G, Medaer R, Raus J, et al. Myelin reactive T cells after T cell vaccination in multiple sclerosis: cytokine profile and depletion by additional immunizations. J Neuroimmunol 2000; 102: 79–84
Stinissen P, Hermans G, Hellings N, et al. Functional characterization of CD8 anti-clonotypic T cells from MS patients treated with T cell vaccination [abstract]. J Neuroimmunol 1998; 90: A564
Zang YCQ, Hong J, Tejada-Simon MV, et al. Th2 immune regulation induced by T cell vaccination in patients with multiple sclerosis. Eur J Immunol 2000; 30: 908–13
Buenafe AC, Tsaknaridis LJ, Spencer L, et al. Specificity of regulatory CD4+CD25+ T cells for self-T cell receptor determinants. J Neurosci Res 2004; 76: 129–40
Vandenbark AA, Culbertson NE, Bartholomew RM, et al. Therapeutic vaccination with a trivalent T cell receptor peptide vaccine restores deficient FoxP3 expression and TCR recognition in subjects with multiple sclerosis. Immunology 2007; 123: 66–78
Hong J, Zang Y, Tejada-Simon MV, et al. Reactivity and regulatory properties of human anti-idiotypic antibodies induced by T cell vaccination. J Immunol 2000; 165: 6858–64
Acknowledgments
The authors wish to thank Ms Eva Niehaus for assistance in preparing the manuscript and acknowledge the support of the Yeshya Horowitz Association (New York, NY, USA), the National Institutes of Health (grant no. NS23221), the Nancy Davis MS Center Without Walls, and the Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Dr Vandenbark has a significant financial interest in Orchestra Therapeutics (formerly the Immune Response Corporation), a company that has a commercial interest in this research and technology. This potential conflict of interest has been reviewed and managed by Oregon Health and Science University and the Veterans Affairs Medical Center (Portland, OR, USA). Dr Abulafia-Lapid has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vandenbark, A.A., Abulafia-Lapid, R. Autologous T-Cell Vaccination for Multiple Sclerosis. BioDrugs 22, 265–273 (2008). https://doi.org/10.2165/00063030-200822040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200822040-00006