Abstract
Background: Clodronic acid, a first-generation bisphosphonate, has been successfully used in the treatment of high bone turnover states, Paget’s disease and osteolytic bone metastases. However, controversies remain over its optimal dosage and method of administration in the treatment of postmenopausal osteoporosis. In this study we aimed to evaluate the effect of clodronic acid treatment for 3 years on bone mineral density (BMD) in women with postmenopausal osteoporosis.
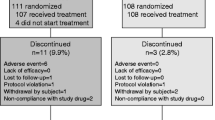
Methods: This was a prospective, open-label, randomised, controlled study that was conducted in an outpatient clinic at the Bone Metabolism Unit of a tertiary referral centre university hospital. Thirty postmenopausal women (age range 48–73 years) with osteoporosis and a control group of 49 osteoporotic women (age range 47–74 years) received randomised therapy. The clodronic acid group of participants received oral doses of clodronic acid 800mg plus elemental calcium 500mg and 400IU of vitamin D daily, while the control group was treated with calcium and vitamin D only. BMD was measured by dual energy x-ray absorptiometry at yearly intervals. Biochemical markers of bone turnover were also measured.
Results: In this clinical study of postmenopausal women with osteoporosis, 36 months of clodronic acid treatment significantly increased average femoral neck BMD by 3.2 ± 2.9%, trochanter BMD by 2.2 ± 2.9% and lumbar spine BMD by 3.1 ± 3%. In the control group, femoral neck, trochanter and lumbar spine BMD decreased by −6 ± 2.7%, −7.3 ± 2.5% and −5.4 ± 2%, respectively (p < 0.01, p < 0.05 and p < 0.05 for clodronic acid vs control, respectively). There was a significant decrease in urinary hydroxyproline (−38.3%) over 3 years in the clodronic acid group compared with baseline (p < 0.05), while no significant change occurred in the control group. Clodronic acid was well tolerated and compliance was good. There were no clinically meaningful differences in the incidence of individual adverse events between the groups.
Conclusion: These results indicate that daily oral administration of clodronic acid 800mg provides benefits to skeletal bone density in osteoporotic postmenopausal women. Calcium and vitamin D supplementation alone did not prevent further bone loss.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and treatment. JAMA 2001; 285: 785–95
Melton LJ III, Chrischilles EA, Cooper C, et al. How many women have osteoporosis? JBMR Anniversary Classic. J Bone Miner Res 2005 May; 20(5): 886–92
Riggs BL, Melton III LJ. The prevention and treatment of osteoporosis. N Eng J Med 1992 Aug 27; 327(9): 620–7
Plosker GL, Goa KL. Clodronate: a review of its pharmacological properties and therapeutic efficacy in resorptive bone diseases. Drugs 1994 June; 47(6): 945–82
McCloskey EV, Dunn JA, Kanis JA, et al. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. B J Haematol 2001 June; 113(4): 1035–43
Kanis JA, Powles T, Paterson AH, et al. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone 1996 Dec; 19(6): 663–7
Lahtinen R, Laakso M, Palva I, et al. for the Finnish Leukemia Group. Randomised placebo controlled multicentre trial of clodronate in multiple myeloma. Lancet 1992 Oct; 340(8827): 1049–52
Paterson A, Powles T, Kanis J, et al. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol 1993 Jan; 11(1): 59–65
Lehenkari P, Kellinsalmi M, Näpänkangas JN, et al. Further insight into the mechanism of action of clodronate: inhibition of the mitochondrial ADP/ATP translocase by a non-hydrolysable, adenine-containing metabolite. Mol Pharmacol 2002 May; 61(5): 1255–62
Väänänen K. Mechanism of osteoclast mediated bone resorption: rationale for the design of new therapeutics. Adv Drug Deliv Rev 2005 May 25; 57(7): 959–71
Itoh F, Aoyagi S, Kusama H, et al. Effects of clodronate and alendronate on local and systemic changes in bone metabolism in rats with adjuvant arthritis. Inflammation 2004 Feb; 28(1): 15–21
Heikkinen JE, Seiander KS, Laitinen K, et al. Short-term intravenous bisphosphonates in prevention of postmenopausal bone loss. J Bone Miner Res 1997 Jan; 12(1): 103–10
Flipponi P, Cristallini S, Policani G, et al. Intermittent versus continuous clodronate administration in postmenopausal women with low bone mass. Bone 2000 Mar; 26(3): 269–74
Flipponi P, Cristallini S, Rizzello E, et al. Cyclical intravenous clodronate in postmenopausal osteoporosis: results of a long-term clinical trial. Bone 1996 Feb; 18(2): 179–84
Giannini S, D’Angelo A, Malvasi L, et al. Effects of one-year cyclical treatment with clodronate on postmenopausal bone loss. Bone 1993 Mar–Apr; 14(2): 137–41
Minaire P, Berard E, Meunier PJ, et al. Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest 1981 Oct; 68(4): 1086–92
Filipponi P, Cristallini S, Policani G, et al. Intermittent versus continuous clodronate administration in postmenopausal women with low bone mass. Bone 2000 Mar; 26(3): 269–74
Haderslev KV, Tjellesen L, Sorensen HA, et al. Effect of cyclical intravenous clodronate therapy on bone mineral density and markers of bone turnover in patients receiving home parenteral nutrition. Am J Clin Nutr 2002 Aug; 76(2): 482–8
Herrala J, Puolijoki H, Liippo K, et al. Clodronate is effective in preventing corticosteroid-induced bone loss among asthmatic patients. Bone 1998 May; 22(5): 577–82
Rossini M, Braga V, Gatti D, et al. Intramuscular clodronate therapy in postmenopausal osteoporosis. Bone 1999 Feb; 24(2): 125–9
Yenson M. Ildrarda kalsiyum tayini. Klinik Biyokimya Laboratuar Kitabi. 6th ed. Istanbul: Beta Basim Yayin DagitimA. S, 1986: 415-8
Switzer BR. Determination of hydroxyproline in tissue. J Nutr Biochem, 1991; 2: 229–31
Yamada S, Aoto Y, Suou T, et al. Urinary hydroxyproline and hydroxylysine excretions in relation to hepatic hydroxyproline content in chronic liver disease. Clin Biochem 1989 Oct; 22(5): 389–93
Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996 Dec 7; 348(9041): 1535–41
Genant HK, Wu CY, Van Kuijk C, et al. Vertebral fracture assessment using a semi quantitative technique. J Bone Miner Res 1993 Sep; 8(9): 1137–48
Ross PD, Davis JW, Vogel JM, et al. A critical review of bone mass and the risk of fractures in osteoporosis. Calcif Tissue Int 1990 Mar; 46(3): 149–61
Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993 Jan 9; 341(8837): 72–5
Hochberg MC, Greenspan S, Wasnich RD, et al. Changes in bone density and turnover explain the reduction in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 2002 Apr; 87(4): 1586–92
Bone HG, Downs RW, Tucci JR, et al. Dose-response relationships for alendronate treatment in osteoporotic elderly women. Alendronate Elderly Osteoporosis Study Centers. J Clin Endocrinol Metab 1997 Jan; 82(1): 265–74
D’Amelio P, Muratore M, Tinelli F, et al. Effect of raloxifene and clodronate on bone density in postmenopausal osteoporotic women. Int J Tissue React 2003; 25(2): 73–8
Giuliani N, Pedrazzoni M, Negri G, et al. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 1998 May; 22(5): 455–61
Mashiba T, Hirano T, Turner CH, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000 Apr; 15(4): 613–20
Giannini S, D’Angelo A, Sartori L, et al. Continuous and cyclical clodronate therapies and bone density in postmenopausal bone loss. Obstet Gynecol 1996 Sep; 88(3): 431–6
Tsai KS, Hsu SH, Yang RS, et al. The effectiveness of cyclic and continuous oral clodronate therapy on bone density and markers in osteopenic postmenopausal women. Calcif Tissue Int 1999 May; 64(5): 384–8
Välimäki MJ, Laitinen K, Patronen A, et al. for the Probone Study Group. Prevention of bone loss by clodronate in early postmenopausal women with vertebral osteopenia: a dose finding study. Osteoporos Int 2002 Dec; 13(12): 937–47
Filipponi P, Pedetti M, Fedeli L, et al. Cyclical clodronate is effective in preventing postmenopausal bone loss: a comparative study with transcutaneous hormone replacement therapy. J Bone Miner Res 1995 May; 10(5): 697–703
Mccloskey E, Selby P, Davies M, et al. Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res 2004 May; 19(5): 728–36
Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998 Dec 23; 280(24): 2077–82
Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000; 11(1): 83–91
Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 1999; 9(5): 461–8
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the mobile study. J Bone Miner Res 2005 Aug; 20(8): 1315–22
De Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Eng J Med 1996 Oct 3; 335(14): 1016–21
Lufkin EG, Argueta R, Whitaker MD, et al. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporos Int 1994 Nov; 4(6): 320–2
Graham DY, Malaty HM. Alendronate gastric ulcers. Aliment Pharmacol Ther 1999 Apr; 13(4): 515–9
Powles TJ, McCloskey E, Paterson AH, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst 1998 May 6; 90(9): 704–8
Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone 2001 Oct; 29(4): 336–43
Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone résorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 2001 Feb; 296(2): 235–42
Delmas PD, Chapuy MC, Vignon E, et al. Long term effects of dichloromethylene diphosphonate in Paget’s disease of bone. J Clin Endocrinol Metab 1982 Apr; 54(4): 837–44
Kanis JA, McCloskey EV, Sirtori P, et al. Rationale for the use of clodronate in osteoporosis. Osteoporos Int 1993; 3(2 Suppl.): S23–8
Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. J Clin Endocrinol Metab 2000 Nov;85(11): 4118–24
Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006 May; 38(5): 617–27
Ettinger B, San Martin J, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004 May; 19(5): 745–51
Acknowledgements
No sources of funding were used to assist in the preparation of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanakol, R., Yarman, S., Bayraktaroglu, T. et al. Clodronic Acid in the Treatment of Postmenopausal Osteoporosis. Clin. Drug Investig. 27, 419–433 (2007). https://doi.org/10.2165/00044011-200727060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200727060-00005