Abstract
Objectives: To investigate the efficacies of two different triple-therapy regimens (standard versus low doses), and the influence of cytochrome P450 enzyme (CYP) genetic polymorphism on these efficacies, in Japanese patients undergoing Helicobacter pylori eradication treatment.
Methods: All patients received 1 week of triple therapy. Patients in group A (low-dose regimen) received omeprazole 40 mg/day + amoxicillin 1500 mg/day + clarithromycin 800 mg/day; patients in group B (standard-dose regimen) received omeprazole 40 mg/day + amoxicillin 2000 mg/day + clarithromycin 1000 mg/day.
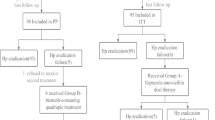
Results: A total of 225 patients (113 in group A and 112 in group B) were randomised to one of the two triple-therapy regimens. The eradication rates were 78.8% (89/113 patients; 95% CI 70.1, 85.9) in group A and 83.0% (93/112 patients; 95% CI 74.8, 89.5) in group B. Genetic polymorphism of CYP2C19, a major metabolic enzyme of omeprazole, did not affect eradication rates, while susceptibility to clarithromycin greatly affected the success of eradication. The cumulative ulcer relapse rate at 24 weeks after endoscopically documented ulcer healing (30 weeks after completion of the drug regimen) was 8.3% for group A and 12.5% for group B (log rank test: p = 0.6248). However, comparison of the cumulative relapse rate of 6.7% in patients after successful H. pylori eradication with the relapse rate of 27.3% in those who failed H. pylori eradication revealed a significant difference in the remission-time curve (log rank test: p = 0.0047). This finding suggested the existence of a relationship between H. pylori eradication failure and ulcer relapse. Both drug regimens were well tolerated. Endoscopically proven reflux esophagitis developed in about 10% of patients after eradication, but was not clinically significant.
Conclusions: One week of triple therapy with a low-dose regimen provides adequate H. pylori eradication in Japanese patients. CYP genetic polymorphism is of minimal clinical significance with both triple-therapy regimens.








Similar content being viewed by others
References
Van der Hulst RW, Rauws EA, Koycu B, et al. Prevention of ulcer recurrence after eradication of Helicobacter pylori: a prospective long-term follow-up study. Gastroenterology 1997; 113: 1082–6
Soll AH. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA 1996; 275: 622–9
Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther 2002; 15: 167–80
Peura D. Helicobacter pylori: rational management options. Am J Med 1998; 105: 424–30
Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther 1995; 9Suppl. 2: 59–69
NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 1994; 272: 65–9
Lind T, Veldhuyzen van Zanten S, Unge P, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter 1996; 1: 138–44
Lind T, Megraud F, Unge P, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 1999; 116: 248–53
Zanten SJ, Bradette M, Farley A, et al. The DU-MACH study: eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther 1999; 13: 289–95
Malfertheiner P, Bayerdorffer E, Diete U, et al. The GU-MACH study: the effect of 1-week omeprazole triple therapy on Helicobacter pylori infection in patients with gastric ulcer. Aliment Pharmacol Ther 1999; 13: 703–12
Japanese Society of Helicobacter Research. The guideline for diagnosis and treatment of Helicobacter pylori infection [in Japanese]. Jpn J Helicobacter Res 2000; 2(Suppl.): 2–12
Japanese Society of Helicobacter Research. The Guideline for diagnosis and treatment of Helicobacter pylori infection 2003 revision [in Japanese]. Jpn J Helicobacter Res 2003; 4(Suppl.): 2–16
Unge P, Kimura K, Sipponen P, et al. Do Japanese and Swedish peptic ulcer patients respond differently to Helicobacter pylori eradication therapies and what are their histological features? Scand J Gastroenterol 2003; 38: 482–90
Janssen MJ, Van Oijen AH, Verbeek AL, et al. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitro-imidazole. Aliment Pharmacol Ther 2001; 15: 613–24
Furuta T, Shirai N, Sugimoto M, et al. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2004; 5: 181–202
Sapone A, Vaira D, Trespidi S, et al. The clinical role of cytochrome p450 genotypes in Helicobacter pylori management. Am J Gastroenterol 2003; 98: 1010–5
Ohara S, Kato M, Asaka M, et al. Studies of 13C-urea breath test for diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol 1998; 33: 6–13
NCCLS. Performance standards for antimicrobial susceptibility testing ninth informational supplement. Pennsylvania: NCCLS, 1999: M100-S9
Committee on assessment of antimicrobial agents: Helicobacter pylori Committee. Establishment of breakpoint of clarithromycin (CAM) and amoxicillin (Amox) for Helicobacter pylori eradication. Tokyo: Japan Society of Chemotherapy, 2000
De Morais SM, Wilkinson GR, Blaisdell J, et al. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 1994; 46(4): 594–8
Japanese Society of Gastroenterology. The guideline for clinical studies on Helicobacter pylori, 1995 [in Japanese]. Tokyo: The Japanese Society of Gastroenterology, 1999
Nakamura M, Spiller RC, Barrett DA, et al. Gastric juice, gastric tissue and blood antibiotic concentrations following omeprazole, amoxicillin and clarithromycin triple therapy. Helicobacter 2003; 8: 294–9
Miwa H, Ohkura R, Murai T, et al. Impact of rabeprazole, a new proton pump inhibitor, in triple therapy for Helicobacter pylori infection: comparison with omeprazole and lansoprazole. Aliment Pharmacol Ther 1999; 13: 741–6
Asaka M, Sugiyama T, Kato M, et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001; 6: 254–61
Furuta T, Shirai N, Watanabe F, et al. Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin Pharmacol Ther 2002; 72: 453–60
Asaka M, Kato M, Sugiyama T, et al. Follow-up survey of a large-scale multicenter, double-blind study of triple therapy with lansoprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. J Gastroenterol 2003; 38: 339–47
Acknowledgements
This study was supported by a research grant from AstraZeneca. The authors have no conflicts of interest directly relevant to the content of this study.
The physicians andmedical institutions involved in this clinical study were: Shunichi Yoshida, Kazuhiko Nishimura, Ikushi Sekimoto, Tadashi Soga and Seiichi Hirano, Department of Gastroenterology, Akashi Municipal Hospital, Akashi; Takeshi Nakamura, Iichiro Tomoda, Kiyotaka Kawaguchi, Takayuki Nada and Keiichi Kiritani, Department of Internal Medicine, Kitano Hospital, Osaka; Mitsuuroko Kubo, Hirohisa Tanimura, Masahide Onoshita and Norihiro Enomoto, Department of Internal Medicine, Osaka Police Hospital, Osaka; Naomi Uemura, Shiro Okamoto and Soichiro Yamamoto, Department of Gastroenterology, Kure Kyousai Hospital, Kure; Tadakazu Sekine, Yoshinari Koyanagi, Hikaru Takahashi and Shigeru Matsui, Department of Gastroenterology, Kawaguchi General Hospital, Kawaguchi; Mitsuru Kaise, Nobuaki Suzuki, Jun Miwa, Department of Gastroenterology, Toshiba Hospital, Tokyo; Hajime Kuwayama, Morio Takahashi, Hiroshi Takada, Yoshiya Matsukawa, Hironobu Takada, Shigeki Oka, Department of Gastroenterology, Koshigaya Hospital of Dokkyo University School of Medicine, Koshigaya; Eiko Sanuki, Hirofumi Kobayashi, Tatsuro Tanimoto, Department of Internal Medicine, Saiseikai Hiroshima Hospital, Hiroshima; Ken Haruma, Koji Sumii, Nobuhiro Tanaka, Yasuhiko Kitadai, Masami Kunihiro, First Department of Internal Medicine, Hiroshima University Medical Hospital, Hiroshima; Tomohiko Shimatani, Masaki Inoue, Yoko Horikawa, General Medicine, Hiroshima University Medical Hospital, Hiroshima; Hiroshi Mieno, Kiminori Ito, Mitsuhiro Mihara, First Department of Internal Medicine, Hiroshima Railway Hospital, Hiroshima; Masahiro Kawanishi, Atsunori Kodoi, Shintaro Takagi, Department of Internal Medicine, Hiroshima Mitsubishi Hospital, Hiroshima.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuwayama, H., Luk, G., Yoshida, S. et al. Efficacy of a Low-Dose Omeprazole-Based Triple-Therapy Regimen for Helicobacter pylori Eradication Independent of Cytochrome P450 Genotype. Clin. Drug Investig. 25, 293–305 (2005). https://doi.org/10.2165/00044011-200525050-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200525050-00002