Abstract
Background and objective
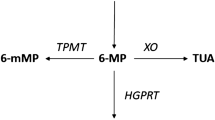
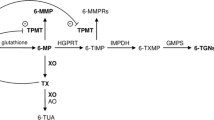
Azathioprine is widely used in the treatment of corticosteroid-dependent and refractory inflammatory bowel disease (IBD). The efficacy of this treatment is based on the production of 6-thioguanine nucleotides, but extremely elevated levels may cause bone marrow suppression. Other azathioprine metabolites, 6-methylmercaptopurine ribonucleotides, are associated with hepatotoxicity. Therapeutic drug monitoring (TDM) may be of help in optimising azathioprine treatment, but data on TDM in established azathioprine therapy are lacking. We therefore measured metabolite levels in a small cohort of patients established on azathioprine therapy.
Patients and methods
6-Thioguanine (6-TGN) and 6-methylmercaptopurine (6-MMP) levels in erythrocytes were measured in 15 IBD outpatients established on azathioprine therapy for at least 3 months at baseline and 1, 4 and 8 weeks after inclusion (mean duration of treatment 28 months; range 7–67 months). Disease activity was evaluated by the Crohn’s Disease Activity Index (Crohn’s disease) or Truelove-Witts (ulcerative colitis) scores. Metabolite levels were measured by modified high-performance liquid chromatography assay (HPLC). Primary outcome measures were 6-TGN and 6-MMP metabolite levels and 95% confidence intervals (CIs). Secondary outcomes were correlations between metabolite levels, drug dose, disease activity and laboratory parameters and compliance.
Results
One patient had active disease during the study period. Eleven of 15 patients (73%) completed the 8-week study period. Dropout reasons were noncompliance in three patients (20%) and intolerance in one patient (7%).
Primary outcomes
At baseline mean 6-TGN levels were 158 (95% CI 113, 203) pmo1/8·108 RBC (red blood cells), steadily increasing over the 8-week study period, but not significantly. Two patients had zero levels. Another two had significantly increasing levels also suggesting noncompliance. Mean 6-MMP levels showed almost a similar pattern. At baseline, levels were 2213 (95% CI 722, 3704) mo1/8·108 RBC.
Secondary outcomes
A correlation was found between all RBC 6-MMP levels and azathioprine dose (mg/kg bodyweight) [r = 0.43, p = 0.001] and also between the 6-MMP/6-TGN ratio and azathioprine dose (mg/kg) [r = 0.36, p = 0.010). There was no correlation between RBC 6-TGN or 6-MMP levels and haematological parameters or disease activity scores. No hepatic, pancreatic or myelotoxicity occurred.
Thirteen of 15 patients (87%) had baseline steady-state 6-TGN levels below the therapeutic threshold of 235 pmol/8·108 RBC. Forty percent (6/15) of our patients were noncompliant; TDM revealed this noncompliance in four of the six patients (27% of all patients).
Conclusion
Our small study demonstrates that TDM may provide insight into individual pharmacokinetics. However, TDM does not seem to be useful in patients with IBD established on azathioprine therapy and without disease activity, although it may be helpful in cases of worsening IBD activity to elucidate noncompliance or inefficient treatment.



Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Lancaster DL, Lennard L, Rowland K, et al. Thioguanine versus mercaptopurine for therapy of childhood lymphoblastic leukaemia: a comparison of haematological toxicity and drug metabolite concentrations. Br J Haematol 1998; 102: 439–43
Bergan S, Rugstad HE, Bentdal O, et al. Kinetics of mercaptopurine and thioguanine nucleotides in renal transplant recipients during azathioprine treatment. Ther Drug Monit 1994; 16: 13–20
Suarez-Almazor M, Spooner C, Belseck E. Azathioprine for rheumatoid arthritis. Cochrane Database Syst Rev 2000; (2): CD001461
Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn disease: a meta-analysis. Ann Intern Med 1995; 123(2): 132–42
Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine and methotrexate. Am J Gastroenterol 1996; 91(3): 423–33
Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989; 111(8): 641–9
Adler DJ, Korelitz BI. The therapeutic efficacy of 6-mercaptopurine in refractory ulcerative colitis. Am J Gastroenterol 1990; 85(6): 717–22
Baert F, Rutgeerts P. 6-Thioguanine: a naked bullet? (or how pharmacogenomics can make old drugs brand new). Inflamm Bowel Dis 2001; 7(3): 190–1
Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol 1992; 43(4): 329–39
Hanauer SB, Meyers SM. Management of Crohn’s disease in adults. Am J Gastroenterol 1997; 92(4): 559–66
Candy S, Wright J, Gerber M, et al. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut 1995; 37: 674–8
Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine: a long-term randomised double-blind study. N Engl J Med 1980; 302: 981–7
Sandborn WJ. Rational dosing of azathioprine and 6-mercaptopurine. Gut 2001; 48: 591–2
Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000; 118: 705–13
Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut 2001; 48(5): 642–6
Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 1996; 39: 401–6
Lowry PW, Franklin CL, Weaver AL, et al. Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut 2001; 49: 665–70
Gupta P, Gokhale R, Kirschner B. 6-Mercaptopurine (6MP) metabolite levels in children with IBD. J Pediatr Gastroenterol Nutr 2001; 33(4): 450–4
Cuffari C, Hunt S, Bayless T. Enhanced bioavailability of azathioprine compared to 6-mercaptopurine therapy in inflammatory bowel disease: correlation with treatment efficacy. Aliment Pharmacol Ther 2000; 14: 1009–14
Dubinsky MC, Hassard PV, Seidman EG, et al. An open-label pilot study using thioguanine as a therapeutic alternative in Crohn’s disease patients resistant to 6-mercaptopurine therapy. Inflamm Bowel Dis 2001; 7(3): 181–9
Derijks LJJ, De Jong DJ, Gilissen LPL, et al. 6-Thioguanine seems promising in azathioprine- or 6-mercaptopurine-intolerant inflammatory bowel disease patients: a short-term safety assessment. Eur J Gastroenterol Hepatol 2003; 15(1): 63–7
Dubinsky MC, Feldman EJ, Abreu MT, et al. Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6-mercaptopurine or azathioprine. Am J Gastroenterol 2003; 98(5): 1058–63
Tiede I, Fritz G, Strand S, et al. CD28-dependent Racl activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003; 111(8): 133–45
Lennard L. TPMT in the treatment of Crohn’s disease with azathioprine. Gut 2002; 51: 143–6
Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000; 188: 1025–30
Kirschner BS. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology 1998; 115(4): 813–21
Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993; 34(8): 1081–5
Dubinsky MC, Vasiliauskas EA, Singh H, et al. 6-Thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology 2003; 125: 298–303
Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. BMJ 1955; 4947: 1041–8
Lennard L, Singleton HJ. High performance liquid Chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-mercaptopurine metabolites in a single sample. J Chromatogr 1992; 583(1): 83–90
Derijks LJJ, Gilissen LPL, Engels, LGJ, et al. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. Ther Drug Monit 2004; 26: 311–8
Pettersson B, Aimer S, Albertioni F, et al. Differences between children and adults in thiopurine methyltransferase activity and metabolite formation during thiopurine therapy: possible role of concomitant methotrexate [abstract]. Ther Drug Monit 2002; 24: 351–8
Reuther LO, Vainer B, Sonne J, et al. Thiopurine methyltransferase (TPMT) genotype distribution in azathioprine-tolerant and -intolerant patients with various disorders: the impact of TPMT genotyping in predicting toxicity. Eur J Clin Pharmacol 2003; 59(11): 797–801
Gearry RB, Barclay ML, Burt MJ, et al. Thiopurine S-methyltransferase (TPMT) genotype does not predict adverse drug reactions to thiopurine drugs in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2003; 118: 395–400
Curvers WL, Derijks LJJ, Stokkers P, et al. No predictive value of TPMT genotyping for leukopenia or hepatotoxicity during azathioprine therapy in inflammatory bowel disease [abstract]. Eur J Gastroenterol Hepatol 2003; 15(12): A29
Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of 6-mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance. Br J Cancer 1995; 72: 1004–6
Webster S, Sanders DS, Lennard L, et al. Azathioprine metabolites in inflammatory bowel disease and incidence of noncompliance [abstract]. Gut 2002; 50 Suppl. II: A71
Acknowledgements
This study received no external funding. The authors have no potential conflicts of interest directly relevant to the contents of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilissen, L.P.L., Derijks, L.J.J., Bos, L.P. et al. Therapeutic Drug Monitoring in Patients with Inflammatory Bowel Disease and Established Azathioprine Therapy. Clin. Drug Investig. 24, 479–486 (2004). https://doi.org/10.2165/00044011-200424080-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200424080-00006