Abstract
Objective: To determine the efficacy and safety profile of 6-month treatment with leflunomide 20mg daily in patients with acute rheumatoid arthritis (RA) receiving treatment from 197 office-based rheumatologists in France.
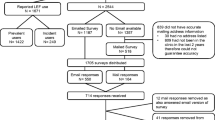
Methods: This open-label, prospective, multicentre study included 378 ambulatory RA patients who received leflunomide at a loading dose of 100mg daily on days 1–3, followed by 20mg daily for 6 months. The primary efficacy endpoint was a >20% response according to the American College of Rheumatology criteria (ACR 20) after 6 months. Secondary efficacy criteria were a ≥50% response (ACR 50) and a ≥70% response (ACR 70), as well as disease activity score (DAS28) responses.
Results: Among the 407 selected patients, 378 patients were included in the study, all of whom were treated with leflunomide. Female patients made up 78.6% of the study population; the mean age was 57.7 ± 12.0 years, and the mean disease duration was 9.7 ± 8.5 years. At 6 months, the ACR 20 response rate was 48.2% (95% confidence interval [CI] 43–53%). ACR 50 and 70 response rates were 25.3% (95% CI 21.0–30.1) and 11.7% (95% CI 8.6–15.4%), respectively. According to the DAS28, 21.8% of patients had a good response, 39.9% a moderate response, and 38.2% were non-responders. The DAS28 response rate was thus 61.8% (95% CI 56.5–66.9%). Mean improvements in tender joint count were -5.6 ± 7.4 (from baseline of 12.2 ± 6.7), in swollen joint count were −4.2 ± 5.7 (from baseline of 9.8 ± 5.8), and in investigator’s global assessment of RA disease activity were-20.2± 25.1 (from baseline of 51.6± 17.1). Treatment-related adverse events caused 15.9% of patients to discontinue the study prematurely. Serious adverse events possibly related to therapy were reported in 2.4% of patients.
Conclusions: This 6-month study carried out under daily routine practice conditions in a typical sample of RA patients showed a favourable efficacy and safety profile for leflunomide 20mg daily. The study confirms the findings of the earlier phase II and III study programme in more selected patient samples.







Similar content being viewed by others
References
Moreland LW, Russell AS, Paulus HE. Management of rheumatoid arthritis: the historical context. J Rheumatol 2001; 28(6): 1431–52
Choy EH, Scott DL. Drug treatment of rheumatic diseases in the 1990s: achievements and future developments. Drugs 1997; 53(3): 337–48
Alldred A, Emery P. Leflunomide: a novel DMARD for the treatment of rheumatoid arthritis. Expert Opin Pharmacother 2001; 2(1): 125–37
Smolen JS, Graninger WB, Emery P. Leflunomide, a new disease-modifying anti-rheumatic drug and the never ending rheumatoid arthritis story. Rheumatology (Oxford) 2000; 39(7): 689–92
Prakash A, Jarvis B. Leflunomide: a review of its use in active rheumatoid arthritis. Drugs 1999; 58(6): 1137–64
Siemasko KF, Chong AS, Williams JW, et al. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation 1996; 61(4): 635–42
Mladenovic V, Domljan Z, Rozman B, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis: results of a randomized, placebo-controlled, phase II study. Arthritis Rheum 1995; 38(11): 1595–603
Weinblatt ME, Kremer JM, Coblyn JS, et al. Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum 1999; 42(7): 1322–8
Strand V, Cohen S, Schiff M, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate: Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med 1999; 159(21): 2542–50
Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet 1999; 353(9149): 259–66
Scott DL, Smolen JS, Kalden JR, et al. Treatment of active rheumatoid arthritis with leflunomide: two year follow up of a double blind, placebo controlled trial versus sulfasalazine. Ann Rheum Dis 2001; 60(10): 913–23
Felson D, Anderson J, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38: 727–35
Harrison BJ, Symmons DP, Barrett EM, et al. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis: American Rheumatism Association. J Rheumatol 1998; 25(12): 2324–30
Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38(1): 44–8
Guillemin F, Braincon S, Pourel J. Measurement of the functional capacity in rheumatoid polyarthritis: a French adaptation of the Health Assessment Questionnaire (HAQ) [in French]. Rev Rheum Mal Osteoartic 1991; 58(6): 459–65
Vrijhoef H, Diederiks J, Spreeuwenberg C, et al. Applying low disease activity criteria using the DAS28 to assess stability in patients with rheumatoid arthritis. Ann Rheum Dis 2003; 62(5): 419–22
Dougados M, Larrey D, Solal-Celigny P, et al. Safety of leflunomide in patients with rheumatoid arthritis: results from the RELIEF study [abstract]. Annual European Congress of Rheumatology (EULAR) 2002; Stockholm Jun 12–15 2002
Emery P, Breedveld FC, Lemmel EM, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000; 39(6): 655–65
Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342(25): 1878–86
Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342(25): 1887–92
Ward MM, Fries JF. Trends in antirheumatic medication use among patients with rheumatoid arthritis, 1981–1996. J Rheumatol 1998; 25(3): 408–16
Pincus T, Marcum SB, Callahan LF. Long-term drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol 1992; 19(12): 1885–94
Mroczkowski PJ, Weinblatt ME, Kremer JM. Methotrexate and leflunomide combination therapy for patients with active rheumatoid arthritis. Clin Exp Rheumatol 1999; 17(6 Suppl. 18): S66–8
Acknowledgements
We would like to acknowledge the cooperation and commitment of all the local general physicians and their staff without whom the present study would not have been possible. Aventis Pharma, Paris, France, funded the study. The authors have provided no information on conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, M., Kabir, M. & Ravaud, P. Short-Term Efficacy and Safety of Leflunomide in the Treatment of Active Rheumatoid Arthritis in Everyday Clinical Use. Clin. Drug Investig. 24, 103–112 (2004). https://doi.org/10.2165/00044011-200424020-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200424020-00005