Abstract
Objective: To assess the average bioequivalence of two formulations of metformin after single-dose administration of each treatment to healthy subjects under fasting conditions by assessing the pharmacokinetic measures of systemic exposure and evaluating the confidence intervals (CIs) for each pharmacokinetic parameter.
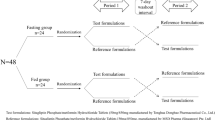
Design: Randomised, comparative, single-dose, open-label, balanced, two-period, two-treatment, crossover study under fasting conditions. Participants: 20 healthy volunteers (ten men and ten women) took part in the study.
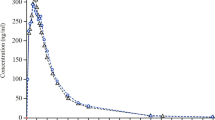
Methods: Subjects were investigated after a single dose of 850mg after a washout period of 7 days. Plasma samples were taken at regular time intervals according to the study protocol for measuring plasma metformin concentrations. Systemic exposure was estimated with the use of pharmacokinetic parameters (area under the curve of the plasma drug concentrations from time zero to the last sampling time [AUC0-36], area under the curve of the plasma drug concentrations from time zero to infinity [AUC0-∞], time to peak drug concentration [tmax], partial area under the concentration-time curve with a cut-off point at the tmax of the reference product [AUCp], peak plasma drug concentration [Cmax], the ratio Cmax/AUC0-∞, and mean residence time [MRT]). The point estimates of pharmacokinetic parameters (geometric means of the ratios test [T]/reference [R] and the 90% CIs for the ratios of geometric means [T]/[R]), estimated by parametric and nonparametric analysis, were used in the statistical analysis.
Results: The point estimates and the 90% CIs after parametric analysis of AUC0-∞ were 0.98 and (0.96–1.21), and after nonparametric analysis were 1.06 and (0.95–1.207), respectively. The two drug products were considered to be bioequivalent and with significant variability across subjects for the pharmacokinetic parameters AUC0–36, AUC0-∞, Cmax and MRT according to analysis of variance of log-transformed data.
Conclusions: The two studied formulations of metformin were found to be bioequivalent. They showed similar extents and rates of absorption and similar exposure. However, analysis of variance of logarithmically transformed data revealed significant variability among individuals in AUC0–36, AUC0-∞ and Cmax, making careful individualisation of the metformin dosage important.



Similar content being viewed by others
Notes
1The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Cusi K, DeFronzo R. Metformin: a review of its metabolic effects. Diabetes Rev 1998; 6(2): 89–131
Bailey C. Metformin: an update. Gen Pharmacol 1993; 24: 1299–309
Turner R, Cull C, Holman R, for the United Kingdom Prospective Diabetes Study Group: United Kingdom prospective diabetes study (UKPDS) 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med 1996; 124 (1 pt 2): 136–45
Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 1996; 30: 359–71
Najib N, Idkaidek N, Beshtawi M, et al. Bioequivalence evaluation of two brands of metformin 500mg tablets (Dialon & Glucophage) in healthy human volunteers. Biopharm Drug Dispos 2002; 23: 301–6
Yuen KH, Wong JW, Billa N, et al. Bioequivalence of a generic metformin tablet preparation. Int J Clin Pharmacol Ther 1999; 37(7): 319–22
Yuen KH, Peh K. Simple high-performance liquid Chromatographic method for the determination of metformin in human plasma. J Chromatogr B Biomed Sci Appl 1988; 710: 243–6
Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products: general considerations. Available from URL: www.fda.gov/cder/guidance/5356fnl.doc [Accessed 2003 Mar]
Hauschke D, Steinjans V, Diletti E, et al. A distribution-free procedure for statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol 1990; 28: 72–3
Sauter R, Steinijans VW, Diletti E, et al. Presentation of results from bioequiavlence studies. Int J Clin Pharmacol Ther Toxicol 1992; 30: S7–13
Wijnand HP. Updates of bioequivalence programs (including statistical power approximation by Student’s t). Comput Methods Programs Biomed 1994; 42: 275–81
Steinijans V, Sauter R, Hauschke D, et al. Reference tables for the intrasubject coefficient of variation in bioequivalence studies. Int J Clin Pharmacol Ther 1995; 33: 427–30
Tucker G, Casey C, Phillips P, et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 1981; 12: 235–46
Luft D, Schmulling R, Eggstein M. Lactic acidosis in biguanide-treated diabetes: a review of 330 cases. Diabetologia 1983; 24: 351–4
Caille G, Lacasse Y, Raymond M. Bioavailability of metformin in tablet form using a new high pressure liquid chromatography assay method. Biopharm Drug Dispos 1993; 14: 257–63
Acknowledgements
The study was funded by the Chemical Pharmaceutical Research Institute, NIHFI, Sofia Bulgaria. The authors have provided no information on conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atanasova, I., Bozhinova, K., Todorova, D. et al. Pharmacokinetics and Comparative Bioavailability of Two Metformin Formulations after Single-Dose Administration in Healthy Subjects. Clin. Drug Investig. 23, 743–749 (2003). https://doi.org/10.2165/00044011-200323110-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200323110-00007