Abstract
Background
An accelerated dose tissue plasminogen activator (tPA) reduces mortality in acute myocardial infarction as compared with streptokinase (SK). Streptokinase is antigenic and will lead to the development of neutralising antibodies that have the potential to limit the effectiveness of repeat SK therapy. Accordingly, tPA is frequently administered to patients with recurrent infarction after SK treatment. Alternatively, one could identify those with high antibody titres and selectively use tPA when resistance was present.
Aim
To determine the marginal cost-effectiveness of various thrombolytic strategies in patients with prior acute myocardial infarction (AMI), including the identification of patients with resistance to SK, using point-of-care testing.
Methods
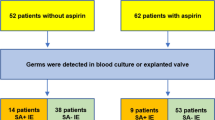
A decision analytical model was used to compare three strategies: repeat SK therapy, tPA to all, or selective tPA use for SK resistance identified using point-of-care testing. The perspective for analysis was that of a Canadian provincial (Ontario) Ministry of Health. Only healthcare costs directly incurred by the insurer were included. Short-term costs were obtained for treatment of AMI and complications. Potential benefits from treatment were estimated only with respect to survival, and are calculated as the additional life-years from the continuing survival of persons who would have died in the first few weeks after AMI but were spared. An incremental cost-effectiveness ratio (CER) was computed as the ratio of these incremental costs and benefits. Therefore, the marginal cost-effectiveness represents dollars (1999 Canadian) per additional short-term (5–6 weeks) survivor.
Results
The use of tPA in all patients with prior AMI resulted in the greatest mortality reduction, whereas the selective use of tPA for those with resistance had the most favourable CER. The selective use of tPA is a cost-effective strategy, with CERs varying from $Can85 964 when 5% are resistant to $Can72 100 when 95% are resistant. The CER improves with increasing levels of resistance. Although more expensive, the administration of tPA to all patients with prior AMI remains cost-effective even when levels of resistance are as low as 5%.
Conclusions
In patients with a prior MI and possible resistance to SK, the use of tPA, whether selectively based on antistreptokinase antibody status or simply given to all, is cost-effective.








Similar content being viewed by others
References
Cairns JA, Fuster V, Kennedy JW, et al. Coronary thrombolysis. Chest 1995; 108(4 Suppl. ): 401S–23S
Fibrinolytic Therapy Trialists’ (FIT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343: 311–21
The International Study Group. In-hospital mortality and clinical course of 20, 891 patients with suspected acute myocardial infarction randomized between alteplase and strepto-kinase with or without heparin. Lancet 1990; 336: 71–5
ISIS-3 Collaborative Group. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41, 299 cases of suspected acute myocardial infarction. Lancet 1992; 339: 753–70
The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329: 673–82
Califf RM, White HD, Van der Werf F, et al. One-year results of the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO-1) Trial. Circulation 1996; 94: 1233–8
Massel D. Canadian consensus conference on coronary thrombolysis: previous SK therapy. Can J Cardiol 1993; 9: 518–20
Dalen JE, Gore JM, Braunwald E, et al. Six- and twelve-month follow-up of the Phase I Thrombolysis in Myocardial Infarction (TIMI) Trial. Am J Cardiol 1988; 62: 179–85
Canadian Consensus Conference on Coronary Thrombolysis. 1994 Recommendations. Can J Cardiol 1994; 10: 522–9
Zijlstra F, Hoorntje JCA, de Boer M-J, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1999; 341: 1413–9
Squire IB, Lawley W, Fletcher S, et al. Humoral and cellular immune responses up to 7.5 years after administration of streptokinase for acute myocardial infarction. Eur Heart J 1999; 20(17): 1245–52
Goel V, Naylor CD. Potential cost effectiveness of intravenous tissue plasminogen activator versus streptokinase for acute myocardial infarction. Can J Cardiol 1992; 8: 31–8
Massel D. Potential cost-effectiveness of tissue plasminogen activator among patients previously treated with strepto-kinase. Can J Cardiol 1999; 15: 173–9
Naylor CD, Jaglal SB. Impact of intravenous thrombolysis on short-term coronary revascularization rates: a meta-analysis. JAMA 1990; 264: 697–702
Mark DB, Hlatky MA, Califf RM, et al. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med 1995; 332: 1418–24
Brieger DB, Mak K-H, White HD, et al. Benefit of early sustained reperfusion in patients with prior myocardial infarction (the GUSTO-1 Trial). Am J Cardiol 1998; 81: 282–7
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17, 187 cases of suspected acute myocardial infarction. Lancet 1988; II: 349–60
Midgette AS, Wong JB, Beshansky JR, et al. Cost-effectiveness of streptokinase for acute myocardial infarction. Med Decis Making 1994; 14(2): 108–17
Kalish SC, Gurwitz JH, Krumholz HM, et al. A cost-effectiveness model of thrombolytic therapy for acute myocardial infarction. J Gen Intern Med 1995; 10: 321–30
Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. BMJ 1998; 316: 1337–43
Krumholz HM, Pasternak RC, Weinstein MC, et al. Cost effectiveness of thrombolytic therapy with streptokinase in elderly patients with suspected acute myocardial infarction. N Engl J Med 1992; 327: 7–13
Califf RM, Woodlief LH, Harrell Jr FE, et al. Selection of thrombolytic therapy for individual patients: development of a clinical model. Am Heart J 1997; 133: 630–9
Fletcher AP, Alkjaersig N, Sherry S. The clearance of heterologous protein from the circulation of normal and immunized man. J Clin Invest 1958; 37: 1306–15
Lynch M, Littler WA, Pentecost BL, et al. Antistreptokinase titres after intravenous streptokinase [letter]. Lancet 1990; 335: 534
Massel D, Turpie AGG, Cairns JA, et al. Previous streptokinase therapy inhibits subsequent streptokinase thrombolysis [abstract]. Circulation 1991; 84(Suppl II): 467
Buchalter MB. Are streptokinase antibodies clinically important?. [editorial]. Br Heart J 1993; 70: 101–2
Lynch M, Littler WA, Pentecost BL, et al. Immunoglobulin response to intravenous streptokinase in acute myocardial infarction. Br Heart J 1991; 66: 139–42
Massel D, Turpie AGG, Gill JB, et al. Development of neutralizing antibodies after 1.5 million units of streptokinase in the treatment of acute myocardial infarction [abstract]. Circulation 1989; 80Suppl II: 11–350
Buchalter MB, Suntharalingam G, Jennings I, et al.: Strepto-kinase resistance: when might streptokinase administration be ineffective? Br Heart J 1992; 68: 449–53
Fears R, Ferres H, Glasgow E, et al. Monitoring of streptokinase resistance titres in acute myocardial infarction patients up to 30 months after giving streptokinase or anistreplase and related studies to measure specific antistreptokinase IgG. Br Heart J 1992; 68: 167–70
Jalihal S, Morris GK. Antistreptokinase titres after intravenous streptokinase. Lancet 1990; 335: 184–5
Patel S, Jalihal S, Dutka DP, et al. Streptokinase neutralisation titres up to 866 days after intravenous streptokinase for acute myocardial infarction. Br Heart J 1993; 70: 1 9-21
Elliott JM, Cross DB, Cederholm-Williams SA, et al. Neutralizing antibodies to streptokinase four years after intravenous thrombolytic therapy. Am J Cardiol 1993; 71: 640–5
Gemmill JD, Hogg KJ, Dunn FG, et al. Pre-dosing antibody levels and efficacy of thrombolytic drugs containing strepto-kinase. Br Heart J 1994; 72: 222–5
AHA Medical/Scientific Statement. ACC/AHA Guidelines for the early management of patients with acute myocardial infarction. Circulation 1990; 82: 664–707
Jahn M. Turnaround time down and sharply, yet clinicians want results faster. Med Lab Observer 1993; 25: 24–37
Bailey TM, Topham TM, Wantz S, et al. Laboratory process improvement through point-of-care testing. Jt Comm J Qual Improv 1997; 23: 362–80
Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. BMJ 1998; 316: 1052–7
Keffer JH. Economic considerations of point-of-care testing. Am J Clin Pathol 1995; 104 Suppl. 1: S107–10
Eddy DM. Cost-effectiveness analysis: a conversation with my father. JAMA 1992; 267: 1669–75
Granata AV, Hillman AL. Competing practice guidelines: using cost-effectiveness analysis to make optimal decisions. Ann Intern Med 1998; 128: 56–63
Ubel PA, DeKay ML, Baron J, et al. Cost-effectiveness analysis in a setting of budget constraints: is it equitable? N Engl J Med 1996; 334: 1174–7
Acknowledgement
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massel, D. Identifying Antistreptokinase Antibodies. Clin. Drug Investig. 22, 837–848 (2002). https://doi.org/10.2165/00044011-200222120-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200222120-00004