Abstract
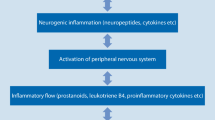
Pain is perceived through activation of the endings of nociceptive afferent nerves by pain-producing substances released from tissue. These nerves in turn activate nociceptive nerve cells in the dorsal horn of the spinal cord through the release of excitatory amino acids and neuropeptides. The activation of the nociceptive afferents can be amplified after repetitive stimulation via the production of nitric oxide, which facilitates the release of excitatory amino acid transmitters.
Pain is one of the cardinal signs of inflammation, the response of tissue to frank or immune damage or infection. The inflammatory response is characterised by vasodilation, oedema and a marked local accumulation of white blood cells. Many intercellular messengers, or cytokines, are responsible for the various stages of inflammation, but tumour necrosis factor seems to be a significant initiator of the response.
The severe pain that accompanies inflammatory disease such as rheumatoid arthritis is caused by the action of pain-producing substances such as kinins on nociceptor neurons sensitised by locally produced prostaglandins, and perhaps sympathomimetics released from sympathetic nerves. The first-line treatment of inflammatory pain is the use of nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit the cyclo-oxygenase enzyme (COX) which produces the hyperalgesic prostaglandins from their substrate arachidonic acid. Since prostaglandins produced in inflammation are formed by an induced COX (COX-2), distinct from that which produces the cytoprotective prostaglandins, NSAIDs that selectively inhibit COX-2 may provide effective therapy without gastrotoxicity Further clinical experience is required to establish the most effective therapy for inflammatory pain.


Similar content being viewed by others
References
Munglani R, Hunt S, Jones J. The spinal cord and chronic pain. Anaesth Rev 1996; 12: 53–6
Dray A. Inflammatory mediators of pain. Br J Anaesth 1995; 75: 125–31
Watkins L, Maier S, Goehler L. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995; 63: 289–302
Emery P. Pharmacology, safety data and therapeutics of COX-2 inhibitors. In: Vane J, Botting J, Botting R, editors. Improved non-steroid anti-inflammatory drugs. COX-2 enzyme inhibitors. London: Kluwer Academic and William Harvey, 1996: 229–41
Willoughby D, Tomlinson A, Gilroy D, et al. Inducible enzymes with special reference to COX-2 in inflammation and apoptosis. In: Vane J, Botting J, Botting R, editors. Improved nonsteroid anti-inflammatory drugs. COX-2 enzyme inhibitors. London: Kluwer Academic and William Harvey, 1996: 67–83
Ferreira S, Lorenzetti B, Correa F. Central and peripheral antialgesic actions of aspirin-like drugs. Eur J Pharmacol 1978; 53: 39–45
Adams D, Lloyd A. Chemokines: leucocyte recruitment and activation cytokines. Lancet 1997; 349: 490–5
Ahluwalia A, Perretti M. B1 receptors as a new inflammatory target. Could this B the 1. Trends Pharmacol Sci 1999; 16: 100–4
Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci 1993; 16: 99–104
Ahluwalia A, Perretti M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1beta in vivo in the mouse. J Immunol 1996; 156: 269–74
Armstrong D, Dry R, Keele C, et al. Observations on chemical excitants of cutaneous pain in man. J Physiol 1953; 120: 326–51
Vane J, Botting R. Analgesic actions of aspirin. In: Vane J, Botting R, editors. Aspirin and other salicylates. London: Chapman and Hall Medical, 1992: 166–212
Boyce S, Rupniak N, Carlson E, et al. Nociception and inflammatory hyperalgesia in B2 receptor knockout mice. Immunopharmacology 1996; 33: 333–5
Ferreira S. Prostaglandins, aspirin-like drugs and analgesia. Nature (New Biol) 1972; 240: 200–3
Bley K, Hunter J, Eglen R, et al. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci 1998; 19: 141–7
Murata T, Ushikubi F, Masuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 1997; 388: 678–82
Poole S, Cunha F, Ferreira S. Hyperalgesia from subcutaneous cytokines. In: Watkins L, Maier S, editors. Cytokines and pain. Basel: Birkhäuser Verlag, 1998: 59–87
Ferreira S. Are macrophages the body’s alarm cells? Agents Actions 1980; 10: 229–30
Vane J, Botting R. The history of anti-inflammatory drugs and their mechanism of action. In: Bazan N, Botting J, Vane J, editors. New targets in inflammation. Inhibitors of COX-2 or adhesion molecules. London: Kluwer Academic and William Harvey, 1996: 1–12
Vane J. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (New Biol) 1971; 231: 232–5
Milton A. Antipyretic actions of aspirin. In: Vane J, Botting R, editors. Aspirin and other salicylates. London: Chapman and Hall Medical, 1992: 213–44
Vane J, Botting R. Mechanism of action of anti-inflammatory drugs: an overview. In: Vane J, Botting J, editors. Selective COX-2 inhibitors. Pharmacology, clinical effects and therapeutic potential. London: Kluwer Academic and William Harvey, 1998: 1–17
Fu J-Y, Masferrer J, Seibert K, et al. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 1990; 265: 16737–40
Xie W, Chapman J, Robertson D, et al. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA 1988; 88: 2692–6
Kujubu D, Fletcher B, Varnum B, et al. TIS 10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 1991; 266: 12866–72
Botting J. Nonsteroidal anti-inflammatory agents. Innovative therapies for rheumatoid arthritis [Internet conference] Jun 15–Sep 30, 1998. Available from Telesymposium Proceedings®, http://www.prous.com/ts. Barcelona: Prous Science
Kamali F. Rofecoxib. Curr Opin CPNS Invest Drugs 1999; 1: 126–31
Wallace J, Chin B. Celecoxib. Curr Opin CPNS Invest Drugs 1999; 1: 132–41
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Botting, R.M., Botting, J.H. Pathogenesis and Mechanisms of Inflammation and Pain. Clin. Drug Investig. 19 (Suppl 2), 1–7 (2000). https://doi.org/10.2165/00044011-200019002-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200019002-00001