Abstract
Objective: Two oral enteric-coated pellet formulations of omeprazole, Pepticum® (test formulation) and Mopral® (reference), were administered to 24 healthy volunteers for 5 days at a daily dose of 20mg omeprazole in order to investigate the comparative bioavailability of the two formulations.
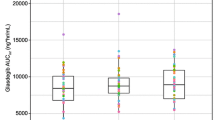
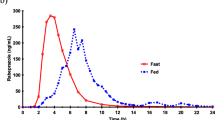
Results: The data obtained in this study demonstrated the bioequivalence of the two formulations. No statistical differences were observed for the area under the plasma concentration-time curve (AUC0-t), the parameter to which the inhibition of acid secretion induced by omeprazole is directly related. Differences observed in maximum plasma drug concentration (Cmax) at day 1 for both formulations were not statistically significant. At steady-state, the differences found in Cmax were associated with a p-value <0.05 with the 90% confidence interval lying between the acceptance range (70 to 140%). Regarding time to reach Cmax(tmax), p < 0.01 was found both after single and repeated doses. In both cases, Pepticum® showed a delay in reaching Cmaxcompared with Mopral®: 2.15 ± 1.11 vs 1.48 ± 0.52h (day 1) and 1.94 ± 0.66 vs 1.31 ± 0.75h (day 5).
Conclusion: This study confirmed the reported increases in AUC and Cmax after repeated administrations, the important intersubject variability and the excellent biological and clinical tolerability of both formulations.
Similar content being viewed by others
References
Fellenius E, Berglindh T, Sachs G. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+,K+)-ATPase. Nature 1981; 290: 159–61
Lindberg P, Nordberg P, Alminger T, et al. The mechanism of action of the gastric acid secretion inhibitor. J Med Chem 1986; 29(8): 1327–9
Brändström A, Lindberg P. Junggren U. Structure activity relationships of substituted benzimidazoles. Scand J Gastroenterol 1985; 108Suppl. 20: 15–22
Oosterhuis B, Jonkman JHG. Omeprazole: pharmacology, pharmacokinetics and interactions. Digestion 1989; 1Suppl. 44: 9–17
Chiverton SG, Howden CW, Burget DW, et al. Omeprazole (20mg) daily given in the morning or evening: a comparison of effects on gastric acidity, and plasma gastrin and omeprazole concentration. Aliment Pharmacol Ther 1992; 6: 103–11
Clissold SP, Campoli-Richards DM. Omeprazole. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer disease and Zollinger-Ellison Syndrome. Drugs 1986; 32: 15–47
Ching MS, Mihaly GW, Angus PW, et al. Oral bioavailability of omeprazole before and after chronic therapy in patients with duodenal ulcer. Br J Clin Pharmacol 1991; 31: 166–70
Anderson T, Olsson R, Regardh CG, et al. Pharmacokinetics of [14C]omeprazole in patients with liver cirrhosis. Clin Pharmacokinet 1993; 24: 71–8
Regardh CG, Andersson T, Lagerström PO, et al. The pharmacokinetics of omeprazole in humans — a study of single intravenous and oral doses. Ther Drug Monit 1990; 12(2): 163–72
Regardh CG, Gabrielsson M. Hoffman KJ, et al. Pharmacokinetics and metabolism of omeprazole in animals and man — an overview. Scand J Gastroenterol 1985; 20Suppl. 108: 79–94
New galenic process for omeprazole containing pellets. Patent application EP519144, 1991
McTavish D, Buckley MMT, Heel RC. Omeprazole. An updated review of its pharmacology and therapeutic use in acid-related disorders. Drugs 1991; 42(1): 138–70
Garg SK, Chugh Y, Tripathi SK, et al. Comparative bioavailability of 2 enteric-coated capsules of omeprazole in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1993; 31: 96–9
Andersson T. Omeprazole drug interaction studies. Clin Pharmacokinet 1991; 21: 195–212
Howden CW, Meredith PA, Forrest JAH, et al. Oral pharmacokinetics of omeprazole. Eur J Clin Pharmacol 1984; 26: 641–3
US Pharmacopeia XXIII, 1791
Eur Pharmacopoeia, 1997, 1748
Steinijans VW, Hauschke D. International harmonization of regulatory bioequivalence requirements. Clin Res Reg Affairs 1993; 10(4): 203–20
Tolman KG, Sanders SW, Buchi KN, et al. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J Clin Gastroenterol 1997; 24(2): 65–70
Prichard PJ, Yeomans ND, Mihaly GW, et al. Omeprazole: a study of its inhibition of gastric pH and oral pharmacokinetics after morning or evening dosage. Gastroenterology 1985; 88: 64–9
Hartman M, Theiss U, Huber R, et al. Twenty four hour intragastric pH profiles and pharmacokinetics following single and repeated oral administration of the proton pump inhibitor pantoprazole in comparison to omeprazole. Aliment Pharmacol Ther 1996; 10: 359–66
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duvauchelle, T., Millerioux, L., Gualano, V. et al. Comparative Bioavailability Study of Two Oral Omeprazole Formulations after Single and Repeated Administrations in Healthy Volunteers. Clin. Drug Investig. 16, 141–149 (1998). https://doi.org/10.2165/00044011-199816020-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199816020-00007