Abstract
Objectives: This paper aimed to provide an overview from published randomised clinical trials of the efficacy and tolerability of lamotrigine monotherapy compared with carbamazepine and phenytoin when initiated in adult patients with newly diagnosed epilepsy.
Design: The review included two double-blind, randomised trials of lamotrigine monotherapy compared with carbamazepine and phenytoin, respectively, and one open randomised trial comparing lamotrigine with carbamazepine. The results of the three trials were pooled for comparison of tolerability.
Setting: Multicentre in Europe.
Patients: Adult patients (>12 years of age) with newly diagnosed partial seizures (with or without secondary generalisation) and primary generalised tonic-clonic seizures (n = 443 patients on lamotrigine, n = 246 on carbamazepine, n = 95 on phenytoin).
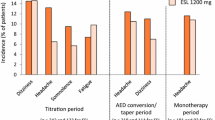
Results: Comparable efficacy was demonstrated between lamotrigine and both carbamazepine or phenytoin. The time to withdrawal survival analysis supported a significant difference in favour of lamotrigine [hazard ratio 1.57 (95% CI 1.07 to 2.31)] in the double-blind trial. Overall, twice the proportion of patients withdrew from carbamazepine or phenytoin because of adverse events (19.1 and 18.9%, respectively) compared with lamotrigine (9.5%). Lamotrigine was particularly well tolerated with regard to adverse effects affecting the central nervous system. Rash was the most common adverse event necessitating discontinuation of each drug, the rates being very similar across treatment groups (6.1% on lamotrigine, 8.9% on carbamazepine, 5.3% on phenytoin). The rate of rash resulting in withdrawal of lamotrigine was clearly related to the dose escalation employed in the different trials during the first month of therapy.
Conclusions: Lamotrigine is an effective monotherapy treatment for adult patients with newly diagnosed epilepsy, and is better tolerated than either carbamazepine or phenytoin monotherapy. The incidence of rash requiring withdrawal of lamotrigine is related to dose escalation (2.2% of patients withdrawing when the recommended escalation was followed).
Similar content being viewed by others
References
Xie X, Lancaster B, Peakman T, et al. Interaction of the anti-epileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch 1995; 430: 437–46
Goa KL, Ross SR, Chrisp P. Lamotrigine. A review of its pharmacological properties and clinical efficacy in epilepsy. Drugs 1993 Jul; 46(1): 152–76
Besag FMC, Wallace SJ, Dulac O, et al. Lamotrigine in the treatment of epilepsy in childhood. J Paediatrics 1995; 127: 991–7
Besag FMC, Dulac O, Alving J, et al. Long-term safety of lamotrigine (Lamictal) in paediatric patients with epilepsy. Seizure 1997 Feb; 6(1): 51–6
Motte J, Trevathan E, Arvidsson JFV, et al. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. N Engl J Med 1987; 337(25): 1807–12
Brodie MJ, Richens A, Yuen AWC, et al. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet 1995; 345: 476–9
Steiner TJ, Silveira C, Yuen AWC, et al. Comparison of lamotrigine (Lamictal) and phenytoin monotherapy in newly diagnosed epilepsy. Epilepsia 1994; 35Suppl. 7: 61
Reunanen M, Dam M, Yuen AWC. A randomised open multicenter comparative trial of lamotrigine and carbamazepine as monotherapy in patients with newly diagnosed or recurrent epilepsy. Epilepsy Res 1996; 23: 149–55
Steiner TJ, Findley LJ, Yuen AWC. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia 1997; 17: 109–12
Kilpatrick ES, Forrest G, Brodie MJ. Concentration-effect and concentration-toxicity relations with lamotrigine: a prospective study. Epilepsia 1996; 37Suppl. 6: 534–8
Brodie MJ, Yuen AWC and the 105 study group. Lamotrigine substitution study: evidence for synergism with sodium valproate? Epilepsy Res 1997; 26: 423–32
Pisani F. Lamotrigine pharmacokinetics. In: Loiseau P, editor. Lamotrigine — a brighter future. International Congress and Symposia, Series 214, Royal Society of Medicine Press, 1996; 3-7
Heller AJ, Chesterman P, Elwes RDC, et al. Phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed adult epilepsy: a randomised comparative monotherapy trial. J Neurol Neurosurg Psych 1995; 58: 44–50
Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, pheobarbital, phenytoin, and primidone in partial and secondarily generalised tonic-clonic seizures. N Engl J Med 1985; 313: 145–51
Mattson RH, Cramer JA, Collins JF and the Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondary generalised tonic-clonic seizures in adults. N Engl J Med 1992; 327: 765–71
Chadwick DW, Shaw MDM, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psych 1984; 47: 642–4
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lynette Mullens, E. Clinical Experience with Lamotrigine Monotherapy in Adults with Newly Diagnosed Epilepsy. Clin. Drug Investig. 16, 125–133 (1998). https://doi.org/10.2165/00044011-199816020-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199816020-00005