Summary
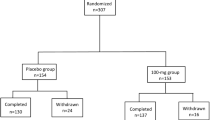
CR FIRST (Sinemet® CR Five Year International Response Fluctuation Study) is a multicentre clinical trial (currently in its fifth year of implementation) designed to test the hypothesis that long term treatment of patients with Parkinson’s disease with a controlled-release formulation of levodopa (Sinemet® CR) will extend the time until the onset of clinical response fluctuations. CR FIRST was designed with sufficient power to detect a clinically significant treatment effect, if one exists. A total of 618 levodopa-natïve patients with Parkinson’s disease, who required therapy with levodopa, were enrolled at 36 sites throughout the world. Patients were randomised to receive 1 tablet twice a day of either immediate-release Sinemet® 25/100 (carbidopa/levodopa) or Sinemet® CR 50/200, then titrated according to response. Patients are being evaluated at regular intervals over 5 years to determine if and when the primary end-point of response fluctuations is attained. At baseline, the cohort represented a moderately disabled patient population, as evidenced by an average Hoehn and Yahr state of 1.9 and an average duration since diagnosis of 2.3 years. As expected, the CR FIRST population had a higher degree of disease severity than patients in the Deprenyl and Tochopherol Antioxidative Therapy of Parkinsonism (DATATOP) cohort. The 2 populations were very similar with regard to demographic characteristics.
Similar content being viewed by others
References
Parkinson Study Group. DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Arch Neurol 1989; 46: 1052–60
Barbeau A. The clinical physiology of side effects in long term L-dopa therapy. Adv Neurol 1974; 5: 347–65
Marsden CD, Parkes JD. ‘On-off’ effects in patients with Parkinson’s disease on chronic levodopa therapy. Lancet 1976; II: 292–6
Sweet RD, McDowell FH. Plasma dopa concentrations and the ‘on-off’ effect after chronic treatment of Parkinson’s disease. Neurology 1974; 24: 953–6
Quinn NP, Levodopa. In: Koller WC, editor. Handbook of Parkinson’s disease. New York: Marcel Dekker; 1987: 317–37
Chase TN, Baronti F, Fabbrini GI, et al. Rationale for continuous dopaminomimetic therapy of Parkinson’s disease. Neurology 1989; 39(2): 7–10
Olanow CW. Oxidation reactions in Parkinson’s disease. Neurology 1990; 40: 32–7
Blin J, Bonnett AM, Agid Y. Does levodopa aggravate Parkinson’s disease? Neurology 1988; 38(9): 1410–6
Quinn NP, Parkes JD, Marsden CD. Control of on/off phenomenon by continuous infusion of levodopa. Neurology 1984; 34: 1131–6
Juncos J, Serrati C, Fabbrini G, et al. Fluctuating levodopa concentrations in Parkinson’s disease. Lancet 1985; II: 440
Cedarbaum JM. The promise and limitations of controlled release oral levodopa administration. Clin Neuropharmacol 1989; 12: 147–66
Marsden CD. Parkinson’s disease. Lancet 1990; 335: 948–52
Duvoisin RC. New strategies in dopaminergic therapy of Parkinson’s disease: the use of a controlled-release formulation. Neurology 1989; 39(2): 4–6
Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 1989; 321(20): 1364–71
Gauthier S, Amyot D. Sustained release antiparkinson agents: controlled release levodopa. Can J Neurol Sci 1992; 19: 153–5
Alba A, Trainor FS, Ritter W, et al. A clinical disability rating for Parkinson’s patients. J Chron Dis 1968; 21: 507–22
Canter CJ, de la Torre R, Mier M. A method of evaluating disability in patients with Parkinson’s disease. J Nerv Ment Dis 1961; 133: 143–7
Fahn S, Elton RL, members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, et al, editors. Recent developments in Parkinson’s disease II. Florham Park, NJ: Macmillan, 1987
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–42
Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML, editors. Third symposium on Parkinson’s disease. Edinburgh, UK: Livingstone, 1969: 152–7
Hunt SM, McEwen J, McKenna SP. Measuring health status. Dover, NH; Croom Helm, 1986
Elandt-Johnson C, Johnson NL. Survival models and data analysis. New York; John Wiley, 1980
Lachin JM, Foulkes MA, Evaluation of sample size and power for analysis of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics 1986; 42: 507–19
Schoenberg BS. Descriptive epidemiology of Parkinson’s disease: disease distribution and hypothesis formulation. Adv Neurol 1986; 45: 277–83
Hoehn MM. Parkinson’s disease: progression and mortality. Adv Neurol 1986; 45: 457–61
DuPont E, Anderson A, Boas J, et al. Sustained-release Madopar HBS compared with standard Madopar in the long term treatment of de novo parkinsonian patients. Acta Neurol Scand 1996; 93: 14–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liss, C., Bush, D., Last, B. et al. CR FIRST. Clin. Drug Invest. 13, 15–22 (1997). https://doi.org/10.2165/00044011-199713010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199713010-00003