Summary
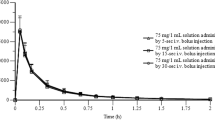
The aim of this study was to demonstrate the bioequivalence of two 100mg sustained-release formulations of diclofenac sodium: the standard formulation, marketed in France as Voltaren LP®, and 3 different batches of a generic formulation marketed in France as Xenid LP®. The study was an open, randomised, 4-way crossover study with 4 treatment periods separated by a washout interval of at least 7 days. All treatments were safe and well tolerated. The diclofenac pharmacokinetic values for Voltaren LP® were similar to those observed in previous studies, with a mean maximum plasma drug concentration (Cmax) of 1827 ± 1146.5 nmol/L, a median time to reach Cmax (tmax) of 5 hours, and an estimated half-life of 3.0 ± 1.7 hours. The mean area under the plasma concentration-time curve up to the last quantifiable concentration (AUC0–t) for Voltaren LP® was 7154 ± 1556.2 nmol/L·h. Overall diclofenac plasma concentrations were much lower after administration of all batches of Xenid LP® than after administration of Voltaren LP®. The mean relative bioavailability from comparison of AUC0–t values for batches A, B and C of Xenid LP® with those for Voltaren LP® was 79.0 ± 22.6%, 59.3 ± 38.5% and 50.2 ± 17.8%, respectively. Cmax was approximately 60 to 74% lower for all batches of Xenid LP® compared with Voltaren LP®. There was inter- and intrasubject variability in the plasma data for all treatments; however, this was more pronounced after administration of Xenid LP®. Coefficient of variation values ranged from 22 to 67% for AUC0–t and Cmax. Comparison of AUC0–t, Cmax and tmax indicated that none of the 3 batches of Xenid LP® were bioequivalent to Voltaren LP®. Additionally, the batches were not bioequivalent when compared with each other, although the diclofenac pharmacokinetics of batches B and C were similar. The low bioavailability of Xenid LP® compared with the standard sustained-release formulation indicates that the 2 formulations are not therapeutically equivalent and, at a dose level of 100mg, Xenid LP® may actually be ineffective as a maintenance treatment for inflammatory disorders, assuming that the amount of systemically available drug is relevant to the clinical effect.
Similar content being viewed by others
References
Ku EC, Lee W, Kothari HV, et al. Effect of diclofenac on the arachidonic acid cascade. Am J Med 1986; 80 Suppl. 4B: 18–23
John VA. The pharmacokinetics and metabolism of diclofenac sodium in animals and in man. In: Haslock I, et al., editors. Diclofenac in the treatment of rheumatic diseases: a conspectus of international experience. Rheumatol Rehabil 1979; 17 Suppl. 2: 22–37
Sioufi A, Stierlin H, Schweizer A, et al. Recent findings concerning clinically relevant pharmacokinetics of diclofen sodium. In: Kass E, editor. Voltaren — new findings. Berne, Stuttgart, Vienna: Hans Huber, 1982: 19–30
Willis JV, Kendall MJ, Flinn RM, et al. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol 1979; 16: 405–10
Voltaren (diclofenac) — twenty years of clinical experience (an update). Basle: Ciba-Geigy Limited, 1994
Gibaldi M, Perrier D. Pharmacokinetics: drugs and the pharmaceutical sciences. 2nd rev. ed. 1982: 15
Raz I, Hussein Z, Samara E, et al. Comparative pharmacokinetic analysis of a novel sustained-release dosage form of diclofenac sodium in healthy subjects. Int J Clin Pharm Ther Toxicol 1988; 26(5): 246–8
Suleiman MS, Najib N, El-Sayed Y, et al. A study on the relative bioavailability of a sustained-release formulation of diclofenac sodium. Int J Clin Pharm Ther Toxicol 1989; 27(6): 276–79
Snedocor GW, Cochran WG. Statistical methods. 8th ed. Iowa State Univ Press, 1982: 217–53
Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharm Biopharm 1987; 15: 657–80
Lehmann EL. Nonparametrics: statistical methods based on ranks. New York: McGraw-Hill, 1975. Chapters 3 and 4
Hasan MM, Najib NM, Muti H. A comparative bioavailability study on two sustained-release formulations of diclofenac sodium following a single dose administration. Int J Clin Pharm Ther Toxicol 1993; 31(8): 387–91
Sørensen K. A long-term investigation of a new antirheumatic drug, diclofenac sodium (Voltaren). Scand J Rheumatol 1978; Suppl. 22: 81–5
Nelson S, Brahim J. An evaluation of the analgesic efficacy of diclofenac potassium, aspirin, and placebo in postoperative dental pain. Todays Ther Trends 1995; 12 Suppl. 1: 3–14
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hooper, I.T., Allen, E., McLaughlin, K. et al. Bioavailability of a Generic Sustained-Release Formulation of Diclofenac Compared with the Standard Sustained-Release Formulation. Clin. Drug Invest. 12, 259–270 (1996). https://doi.org/10.2165/00044011-199612050-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199612050-00005