Summary
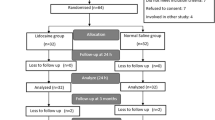
In order to determine the efficacy of lornoxicam in postoperative pain, an observer-blind, placebo-controlled, multiple-dose study was conducted. 80 patients (26 female, 54 male, aged 24 to 61 years) with moderate to severe pain after laminectomy were randomised to receive lornoxicam 4 or 8mg intravenously, pethidine 50mg intravenously or isotonic distilled water (placebo) at their first request for analgesia. Efficacy was determined by the time between the first dose and the first request for remedication, pain intensity differences from baseline (PID) measured by verbal rating scales, the total number of doses required over 24 hours, and patients’ overall impression of efficacy. The median time to remedication was 35 minutes for placebo, 38 minutes for lornoxicam 4mg, 100 minutes for lornoxicam 8mg and 75 minutes for pethidine. Mean PID values were −0.5 for placebo, −0.6 and −0.8 for lornoxicam 4 and 8mg, respectively, and −0.7 for pethidine. Patients receiving lornoxicam 8mg required a mean of 2.6 doses over 24 hours, whilst other groups required 3 doses. There was a consistent trend towards superior efficacy with lornoxicam 8mg and pethidine 50mg, followed by lornoxicam 4mg, then placebo; this was reflected in patients’ overall ratings of ‘good’ for lornoxicam 8mg and pethidine, ‘good to fair’ for lornoxicam 4mg, and ‘fair’ for placebo. Two patients in the lornoxicam 8mg group and 4 in the pethidine group reported adverse events, mostly mild to moderate nausea. One patient in the pethidine group discontinued treatment because of bradycardia. In summary, lornoxicam 8mg intravenously was as effective as pethidine 50mg intravenously in relieving moderate to severe postlaminectomy pain, and had few adverse events.
Similar content being viewed by others
References
Brune K, Menzel-Soglowek S, Zeilhofer HU. Differential analgesic effects of aspirin-like drugs. Drugs 1992; 44 Suppl. 5: 52–9
Catania A, Arnold J, Macaluso A, et al. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc Natl Acad Sci USA 1991; 88: 8544–7
Okuyama S, Aihara H. The mode of action of analgesic drugs in adjuvant arthritic rats as an experimental model of chronic inflammatory pain: possible central analgesic action of acidic nonsteroidal antiinflammatory drugs. Jpn J Pharmacol 1984; 35: 95–103
Hodsman NBA, Burns J, Blyth A, et al. The morphine sparing effects of diclofenac sodium after surgery. Anaesthesia 1987; 42: 1005
Moote C. Efficacy of nonsteroidal anti-inflammatory drugs in the management of postoperative pain. Drugs 1992; 44 Suppl. 5: 14–30
Stubbs DF, Freeman BE, Johnson JH. Efficacy of flurbiprofen for nonarthritic pain. Curr Ther Res 1989; 46: 1179–95
Todd PA, Clissold SP. Naproxen: a reappraisal of its pharmacology and therapeutic use in rheumatic diseases and pain states. Drugs 1990; 40: 91–137
Buckley M-T, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 1990; 39: 86–109
Camu F, Van Lersberghe C, Lauwers MH. Cardiovascular risks and benefits of perioperative nonsteroidal anti-inflammatory drug treatment. Drugs 1992; 44 Suppl. 5: 42–51
Kahlet H, Dahl JB. Are perioperative nonsteroidal anti-inflammatory drugs ulcerogenic in the short term? Drugs 1992; 44 Suppl. 5: 38–41
Kenny GNC. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs 1992; 44 Suppl. 5: 31–7
Stroissnig H, Frenzel W. Lornoxicam. A novel highly potent anti-inflammatory and analgesic agent. Clinical Investigator’s Brochure. 6th ed. Vienna: Hafslund Nycomed Pharma AG, 1992
Ross DA, Drasner K, Weinstein PR, et al. Use of intrathecally administered morphine in the treatment of postoperative pain after lumbar spinal surgery: a prospective, double-blind, placebo-controlled study. Neurosurgery 1991; 28: 700–4
Ozuna J, Burchiel KJ, Pencek T. Routine use of epidural morphine in patients following lumbar spine surgery. Clin J Pain 1988; 4: 209–12
Rechtine GR, Love LC. Postoperative laminectomy pain control using bupivacaine and epidural morphine. Orthopedics 1986; 9: 1711–2
Rechtine GR, Reinert CM. The use of epidural morphine to decrease postoperative pain in patients undergoing lumbal laminectomy. J Bone Joint Surg 1984; 66: 113–6
King JS. Dexamethasone — a helpful adjunct in management after lumbar discectomy. Neurosurgery 1984; 14: 697–700
Max MB, Portenoy RK, Laska EM, editors. Advances in pain research and therapy: the design of analgesic clinical trials. Vol. 18. New York: Raven Press, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenow, D.E., van Krieken, F., Stolke, D. et al. Intravenous Administration of Lornoxicam, a New NSAID, and Pethidine for Postoperative Pain. Clin. Drug Invest. 11, 11–19 (1996). https://doi.org/10.2165/00044011-199611010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199611010-00002