Abstract
The two most common causes of hypercalcemia are primary hyperparathyroidism and neoplastic disease. Parathyroidectomy is the only curative intervention for the former condition. In the rare cases of patients with primary hyperparathyroidism who present with clinical symptoms due to their hypercalcemia, pharmacological treatment may be required. Fluid repletion and intravenous (IV) administration of bisphosphonates are recommended in the literature. Calcium receptor agonists (calcimimetic agents) are at the present time only available for use within clinical trials.
Cancer patients usually present with symptoms of hypercalcemia. Rapid institution of antihypercalcemic treatment is essential in preventing life-threatening deterioration. Fluid repletion and administration of bisphosphonates are the treatment mainstays in hypercalcemia of malignancy. Five bisphosphonates are currently licensed in Europe for treatment of tumor-associated hypercalcemia: etidronate, clodronate, pamidronate, ibandronate, and zoledronate. In the US, pamidronate and zoledronate are licensed for use in this indication.
Bisphosphonates containing nitrogen atoms (e.g. pamidronate, ibandronate, and zoledronate) are more potent than those without (e.g. etidronate, clodronate, and tiludronate). In patients with malignant hypercalcemia, the efficacy of the individual bisphosphonate depends on dose administered and initial serum calcium concentration. At present, pamidronate has been studied in the greatest number of investigations and in the largest number of patients. In the literature, the efficacy of pamidronate in restoring normocalcemia ranges between 40% and 100%, depending on the dose used and baseline serum calcium concentration. More recently, one study reported that pamidronate was inferior to zoledronate. In this study, the duration of response was also longer in the two zoledronate groups (30 and 40 days) than in the pamidronate group (17 days).
The most serious adverse events of bisphosphonates concern renal function. Increases in serum creatinine levels have been more frequently reported following treatment of tumor-associated hypercalcemia with etidronate (8%) and clodronate (5%) than with the nitrogen-containing bisphosphonates pamidronate (2%) and ibandronate (1%). The frequency of increases in serum creatinine levels following treatment with zoledronate is difficult to estimate. Administration of the nitrogen-containing bisphosphonates has been associated with transient (usually mild) fever, lymphocytopenia, malaise, and myalgias. These events occur within 36 hours of the first dose and are self-limiting. Hypocalcemia occurs in up to 50% of patients treated with bisphosphonates for hypercalcemia of malignancy, although symptomatic hypocalcemia is rare.
The toxicity and low efficacy of plicamycin (mithramycin) mean that use of this agent should be restricted to patients with hypercalcemia of malignancy who fail to respond to IV bisphosphonates. Calcitonin is characterized by good tolerability but poor efficacy in normalizing the serum calcium level. However, a major advantage of calcitonin is the acute onset of the hypocalcemic effect, which contrasts with the delayed but more pronounced effect of bisphosphonates. Combination calcitonin and bisphosphonate treatment may therefore be of value when rapid reduction of serum calcium is warranted. Gallium nitrate may be a valuable treatment for hypercalcemia of malignancy. It is characterized by high efficacy and few adverse events apart from renal toxicity (10% of cases). However, data are very limited and further trials are necessary.













Similar content being viewed by others
References
Laposata M. SI unit conversion guide. Boston (MA): NEJM Books, 1992
Shane E. Hypercalcemia: pathogenesis, clinical manifestations, differential diagnosis, and management. In: Farus MJ, editor. Primer on the metabolic bone disease and disorders of mineral metabolism: an official publication of The American Society for Bone and Mineral Research. 4th ed. Philadelphia (PA): Lippincott, 1999: 183–7
Heath DA. Primary hyperparathyroidism: clinical presentation and factors influencing clinical management. Endocrinol Metab Clin North Am 1989; 18: 631–46
Mundy GR, Cove DH, Fisken R, et al. Primary hyperparathyroidism: changes in the pattern of clinical presentation. Lancet 1980; I: 1317–20
Vassilopoulou-Sellin R, Newman BM, Taylor SH, et al. Incidence of hypercalcemia in patients with malignancy referred to a comprehensive cancer center. Cancer 1993; 71(4): 1309–12
Pecherstorfer M, Schilling T, Blind E, et al. Parathyroid hormone related protein and life expectancy in hypercalcemic cancer patients. J Clin Endocrinol Metab 1994; 78(5): 1268–70
Ralston SH, Gallacher SJ, Patel U, et al. Cancer-associated hypercalcemia: morbidity and mortality: clinical experience in 126 treated patients. Ann Intern Med 1990; 112: 499–504
Otsuka F, Hayakawa N, Ogura T, et al. A case of primary hyperparathyroidism accompanying multiple myeloma. Endocr J 1997; 44: 105–9
Albes B, Bazex J, Bayle-Lebey P, et al. Primary hyperparathyroidism and cutaneous T-cell lymphoma: fortuitous association? Dermatology 2001; 203: 162–4
Hutchesson AC, Bundred NJ, Ratcliffe WA. Survival in hypercalcemic cancer patients with co-existing primary hyperparathyroidism. Postgrad Med J 1995; 71: 28–31
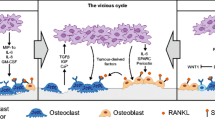
Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93(2): 165–76
Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis: inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 1998; 95(7): 3597–602
Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos Int 2000; 11: 905–13
Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397(6717): 315–23
Hsu H, Lacey DL, Dunstan CR, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A 1999; 96(7): 3540–5
Goltzmann D. Osteolysis and cancer. J Clin Invest 2001; 107: 1219–20
Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-?.B ligand and osteoprotegerin: potential indications for the pathogenesis and treatment of malignant bone disease. Cancer 2001; 92: 460–70
Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999; 20: 345–57
Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89(2): 309–19
Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a common link between osteoclastogenesis, lymph node formation and lymphocytic development. Immunol Cell Biol 1999; 77: 188–93
Hofbauer LC, Khosla S, Dunstan CR, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Mineral Res 2000; 15: 2–12
Roodman DG. Advances in bone biology: the osteoclast. Endocr Rev 1996; 17(4): 308–32
Parfitt AM, Mundy GR, Roodman GD, et al. A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res 1996; 11(2): 150–9
Jüppner H, Brown M, Kronenberg H. Parathyroid hormone. In: Farus MJ, editor. Primer on the metabolic bone disease and disorders of mineral metabolism: an official publication of The American Society for Bone and Mineral Research. 4th ed. Philadelphia (PA): Lippincott, 1999: 80–7
Kovacs CS, Lanske B, Hunzelman JL, et al. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A 1996; 93(26): 15233–8
Uemura H, Yasui T, Yoneda N, et al. Measurement of N- and C-terminal-region fragments of parathyroid hormone-related peptide in milk from lactating women and investigation of the relationship of their concentrations to calcium in milk. J Endocrinol 1997; 153(3): 445–51
Thiebaud D, Janisch S, Koelbl H, et al. Direct evidence of a parathyroid related protein gradient between the mother and the newborn in humans. Bone Miner 1993; 23(3): 213–21
Wysolmerski JJ, Stewart AF. The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu Rev Physiol 1998; 60: 431–60
Bilezikian JP. Primary hyperparathyroidism. In: Farus MJ, editor. Primer on the metabolic bone disease and disorders of mineral metabolism: an official publication of The American Society for Bone and Mineral Research. 4th ed. Philadelphia (PA): Lippincott, 1999: 187–91
Hobbs MR, Heath III H. Familial hyperparathyroid syndromes. In: Farus MJ, editor. Primer on the metabolic bone disease and disorders of mineral metabolism: an official publication of The American Society for Bone and Mineral Research. 4th ed. Philadelphia (PA): Lippincott, 1999: 192–5
Pecherstorfer M, Zimmer-Roth I, Schilling T, et al. The diagnostic value of urinary pyridinium cross-links of collagen, serum total alkaline phosphatase, and urinary calcium excretion in neoplastic bone disease. J Clin Endocrinol Metab 1995; 80(1): 97–103
Mundy GR, Guise TA. Hypercalcemia of malignancy. Am J Med 1997; 103: 134–45
Kremer R, Shustik C, Tabak T, et al. Parathyroid hormone-related peptide in hematologic malignancies. Am J Med 1996; 100(4): 406–11
Grill V, Martin TJ. Hypercalcemia. In: Rubens RD, Mundy GR, editors. Cancer and the skeleton. London: Martin Dunitz, 2000: 75–89
Schilling T, Pecherstorfer M, Blind E, et al. Parathyroid hormone-related protein (PTHrP) does not regulate 1,25 Dihydroxy vitamin D levels in hypercalcemia of malignancy. J Clin Endocrinol Metab 1993; 76: 801–3
Croucher PI, Shipman CM, Lippitt J, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 2001; 98: 3534–40
Zhang J, Dai J, Qi Y, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Investig 2001; 107: 1235–44
Nosaka K, Miyamoto T, Sakai T, et al. Mechanism of hypercalcemia in adult T-cell leukemia: overexpression of receptor activator of nuclear factor? B ligand on adult T-cell leukemia cells. Blood 2002; 99: 634–40
Mundy GR. Calcium homeostasis: hypercalcemia and hypocalcemia. 2nd ed. London: Martin Dunitz, 1990
Insogna KL, Dreyer BE, Mitnich M, et al. Enhanced production rate of 1,25 hydroxyvitamin D in sarcoidosis. J Clin Endocrinol Metab 1988; 66: 72–5
Playford EG, Bansal AS, Looke DFM, et al. Hypercalcemia and elevated 1,25(OH)2D3 levels associated with disseminated mycobacterium avis infection in AIDS. J Infect 2001; 42: 157–8
D’Souza-Li L, Yang B, Canaff L, et al. Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalcuric hypercalcemia and autosomal dominant hypocalcemia. J Clin Endocrinol Metab 2002; 87: 1309–18
El-Hajj Fuleihan G. Familial benign hypocalciuric hypercalcemia. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop: asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Mineral Res 2002; 17Suppl. 2: N51–6
Heath III H. Familial benign (hypocalcuric) hypercalcemia: a troublesome mimic of hyperparathyroidism. Endocrinol Metab Clin North Am 1989; 18: 723–40
Bell NH. Osteomalacia and rickets. In Becker KL, editor. Principles and practice of endocrinology. 2nd ed. Philadelphia (PA): JB Lippincott, 1995: 566
Hatake K, Uwai M, Ohtsuki T, et al. Rare but important adverse effects of all-trans retinoic acid in acute promyelocytic leukemia and their management. Int J Hematol 1997; 66: 13–9
Nikolic-Tomasevic Z, Jelic S, Popov I, et al. Tumor “flare” hypercalcemia-an additional indication for bisphosphonates? Oncology 2001; 60: 123–6
Takata S, Yasui N. Disuse osteoporosis. J Med Invest 2001; 48: 147–56
Ziegler R. Hypercalcemic crisis. J Am Soc Nephrol 2001; 12Suppl. 17: S3–9
Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Miner Res 2002; 17Suppl. 2: N1–162
Tal A, Graves L. Intravenous pamidronate for hypercalcemia of primary hyperparathyroidism. South Med J 1996; 89: 637–40
Tisell LE, Hedback G, Jansson S, et al. Management of hyperparathyroid patients with grave hypercalcemia. World J Surg 1991; 15: 730–7
Kotzmann H, Svoboda T, Bernecker P, et al.Disodium pamidronate (APD) in therapy of hypercalcemia in primary hyperparathyroidism. Wien Klin Wochenschr 1994; 106: 422–5
Ishimura E, Miki T, Harada K, et al. Effect of aminohydroxypropylidene diphosphonate on the bone metabolism of patients with parathyroid adenoma. Horm Metab Res 1993; 25: 493–7
Christensen JH, Kristiansen JH. Combination therapy with pamidronate and calcitonin in hypercalcemic crisis caused by primary hyperparathyroidism. Ugeskr Laeger 1992; 16: 3341–2
Janson S, Tisell LE, Linstedt G, et al. Disodium pamidronate in the preoperative management of hypercalcemia in patients with primary hyperparathyroidism. Surgery 1991; 110: 480–6
Evans RA. Aminohydroxypropylidene diphosphonate treatment of hypercalcemic crisis due to primary hyperparathyroidism. Aust N Z J Med 1987; 17: 58–9
Mundy GR, Wilkinson R, Heath DA. Comparative study of medical therapy for hypercalcemia of malignancy. Am J Med 1983; 74: 421–32
Van Breukelen FJ, Bijvoet OL, Frijlink WB, et al. Efficacy of amino-hydroxypropylidene bisphosphonate in hypercalcemia: observations on regulation of serum calcium. Calcif Tissue Int 1982; 34: 321–7
Hamdy NA, Gray RE, McCloskey E, et al. Clodronate in the medical management of hyperparathyroidism. Bone 1987; 8Suppl. 1: S69–77
Shane E, Jacobs TP, Siris ES, et al. Therapy of hypercalcemia due to parathyroid carcinoma with intravenous dichloromethylene diphosphonate. Am J Med 1982; 72: 939–44
Silverberg SJ, Bone III HG, Marriott TB, et al. Short term inhibition of parathyroid hormone secretion by a calcium-receptor agonist in patients with primary hyperparathyroidism. N Engl J Med 1997; 337: 1506–10
Antoniucci DM, Shoback D. Calcimimetics in the treatment of primary hypeparathyroidism. In: Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Miner Res 2002; 17Suppl. 2: N141–5
Peacock M, Bilezikian JP, Turner SA, et al. Long-term treatment with the calcimimetic AMG 073 in patients with primary hyperparathyroidism [abstract]. Proceedings of the Annual Meeting of The Endocrine Society; San Francisco; 2002 Jun 19–22: 92, OR 21-1
Sleeboom HP, Bijvoet OLM, Van Oosterom AT, et al. Comparison of intravenous (3-amino-1-hydroxypropylidene)-1-1-bisphosphonate and volume repletion in tumour-induced hypercalcemia. Lancet 1983; II: 239–43
Fleisch H. Bisphosphonates: pharmacology and use in the treatment of tumor-induced hypercalcemia and metastatic bone loss. Drugs 1991; 42(6): 919–44
Zojer N, Keck AV, Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcemia of malignancy. Drug Saf 1999; 21: 389–406
Flores JF, Singer FR, Rude RK. Effectiveness of a 24-hour infusion of etidronate disodium in the treatment of hypercalcemia of malignant disease. Miner Electrolyte Metab 1991; 17: 390–5
Body JJ, Lortholary A, Romieu G, et al. A dose-finding study of zoledronate in hypercalcemic cancer patients. J Bone Miner Res 1999; 14: 1557–61
Major P, Lortholary J, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19: 558–67
Hasling C, Charles P, Mosekilde L. Etidronate disodium in the management of malignancy related hypercalcemia. Am J Med 1987; 82Suppl. 2A: 51–4
Singer FR, Ritch PS, Lad TE, et al. Treatment of hypercalcemia of malignancy with intravenous etidronate: a controlled multicenter study. Arch Intern Med 1991; 151: 471–6
Warrell RP, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol 1991; 9(8): 1467–75
Gucalp R, Ritch P, Wiernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol 1992; 10(1): 134–42
Cohen AI, Koeller J, Davis TE, et al. IV dichloromethylene diphosphonate in cancer associated hypercalcemia: a phase I–II evaluation. Cancer Treat Rep 1981; 65(7–8): 651–3
Jacobs TP, Siris ES, Bilezikian JP, et al. Hypercalcemia of malignancy: treatment with intravenous dichloromethylene diphosphonate. Ann Intern Med 1981; 94: 312–6
Urwin GH, Yates AJP, Gray RES, et al. Treatment of hypercalcemia of malignancy with intravenous clodronate. Bone 1987; 8Suppl. 1: S43–51
Harjung H, Fritze D. Results of treating tumour-induced hypercalcemia with clodronate alone. Dtsch Med Wochenschr 1990; 115: 48–52
Rotstein S, Glas U, Eriksson M, et al. Intravenous clodronate for the treatment of hypercalcemia in breast cancer patients with bone metastases: a prospective randomised placebo-controlled study. Eur J Cancer 1992; 28A(4/5): 890–3
O’Rourke NP, McCloskey EV, Vasikaran S, et al. Effective treatment of malignant hypercalcemia with a single intravenous infusion of clodronate. Br J Cancer 1993; 67(3): 560–3
Purohit OP, Radstone CR, Anthony C, et al. A randomized double-blind comparison of intravenous pamidronate and clodronate in the hypercalcemia of malignancy. Br J Cancer 1995; 72: 1289–93
Ralston SH, Dryburgh FJ, Cowan RA, et al. Comparison of aminohydroxypropylidene diphosphonate, mithramycin, and corticosteroids/calcitonin in the treatment of cancer-associated hypercalcemia. Lancet 1985; II: 907–10
Thiebaud D, Jaeger P, Jacquet AF, et al. A single day treatment of tumor-induced hypercalcemia by intravenous amino-hydroxypropylidene bisphosphonate. J Bone Miner Res 1986; 1(6): 555–62
Body JJ, Pot M, Borkowski A, et al. Dose/response study of aminohydroxypropylidene bisphosphonate in tumor-associated hypercalcemia. Am J Med 1987; 82: 957–63
Cantwell BM, Harris AL. Effect of single high dose infusions of aminohydroxypropylidene diphosphonate on hypercalcemia caused by cancer. BMJ 1987; 294: 467–9
Coleman RE, Rubens RD. 3(amino-1,1-hydroxypropylidene) bisphosphonate (APD) for hypercalcemia of breast cancer. Br J Cancer 1987; 56: 465–9
Yates AJP, Murray RML, Jerums GJ, et al. A comparison of single and multiple intravenous infusions of 3-amino-1-hydroxypropylidene-1,1-bisphosphonate (APD) in the treatment of hypercalcemia of malignancy. Aust N Z J Med 1987; 17: 387–91
Morton AR, Cantrill JA, Craig AE, et al. Single dose versus daily intravenous aminohydroxypropylidene bisphosphonate (APD) for the hypercalcemia of malignancy. BMJ 1988; 296: 811–4
Thiebaud D, Jaeger P, Jacquet AF, et al. Dose-response in the treatment of hypercalcemia of malignancy by a single infusion of the bisphosphonate AHPrBP. J Clin Oncol 1988; 6(5): 762–8
Body JJ, Magritte A, Seraj F, et al. Aminohydroxypropylidene bisphosphonate (APD) treatment for tumor-associated hypercalcemia: a randomized comparison between a 3-day treatment and single 24-hour infusions. J Bone Miner Res 1989; 4(6): 923–8
Davis JRE, Heath DA. Comparison of different dose regimens of aminohydroxypropylidene-1, 1-bisphosphonate (APD) in hypercalcemia of malignancy. Br J Clin Pharmacol 1989; 28: 269–74
Gallacher SJ, Ralston SH, Patel U, et al. Side effects of pamidronate. Lancet 1989; II: 42–3
Mannix KA, Carmichael J, Harris AL, et al. Single high-dose (45mg) infusions of aminohydroxypropylidene diphosphonate for severe malignant hypercalcemia. Cancer 1989; 64: 1358–61
Sawyer N, Newstead C, Drummond A, et al. One-shot high-dose pamidronate disodium (APD): effective, simple treatment for hypercalcemia in haematological malignancy. Clin Lab Haematol 1989; 11: 179–84
Sawyer N, Newstead C, Drummond A, et al. Fast (4-h) or slow (24-h) infusions of pamidronate disodium (aminohydroxypropylidene diphosphonate (APD)) as single shot treatment for hypercalcemia. Bone Miner 1990; 9: 122–8
Pecherstorfer M, Janisch S, Marosi C, et al. Treatment of cancer-associated hypercalcemia with pamidronate. Klin Wochenschr 1991; 69: 690–5
Gallacher SJ, Ralston SH, Fraser WD, et al. A comparison of low versus high dose pamidronate in cancer-associated hypercalcemia. Bone Miner 1991; 15(3): 249–56
Dodwell DJ, Howell A, Morton AR, et al. Infusion rate and pharmacokinetics of intravenous pamidronate in the treatment of tumour-induced hypercalcemia. Postgrad Med J 1992; 68: 434–9
Ostenstad B, Andersen OK. Disodium pamidronate versus mithramycin in the management of tumour-associated hypercalcemia. Acta Oncol 1992; 31(8): 861–4
Thürlimann B, Waldburger R, Senn HJ, et al. Plicamycin and pamidronate in symptomatic tumor-related hypercalcemia: a prospective randomized crossover trial. Ann Oncol 1992; 3: 619–23
Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med 1993; 95: 297–304
Gucalp R, Theriault R, Gill I, et al. Treatment of cancer-associated hypercalcemia: double blind comparison of rapid and slow intravenous infusion regimens of pamidronate disodium and saline alone. Arch Intern Med 1994; 154: 1935–44
Zysset E, Ammann P, Jenzer A, et al. Comparison of a rapid (2-h) versus a slow (24-h) infusion of alendronate in the treatment of hypercalcemia of malignancy. Bone Miner 1992; 18: 237–49
Nussbaum SR, Warrell RP, Rude R, et al. Dose-response study of alendronate sodium for the treatment of cancer-associated hypercalcemia. J Clin Oncol 1993; 11(8): 1618–23
Wüster C, Schöter KH, Thiebaud D, et al. Methylpentylaminopropylidenebisphosphonate (BM 21.0955): a new potent and safe bisphosphonate for the treatment of cancer-associated hypercalcemia. Bone Miner 1993; 22: 77–85
Pecherstorfer M, Herrmann Z, Body JJ, et al. Randomized phase II trial comparing different doses of the bisphosphonate ibandronate in the treatment of hypercalcemia of malignancy. J Clin Oncol 1996; 14(1): 268–76
Ralston SH, Thiebaud D, Herrmann Z, et al. Dose-response study of ibandronate in the treatment of cancer-associated hypercalcemia. Br J Cancer 1997; 75(2): 295–300
Dumon JC, Magritte A, Body JJ. Efficacy and safety of the bisphosphonate tiludronate for the treatment of tumor-associated hypercalcemia. Bone Miner 1991; 15: 257–66
O’Rourke NP, McCloskey EV, Rosini S, et al. Treatment of malignant hypercalcemia with aminohexane bisphosphonate (neridronate). Br J Cancer 1994; 69: 914–7
Van Breukelen FJM, Bijovoet OLM, Frijlink WB, et al. Efficacy of aminohydroxypropylidene bisphosphonate in hypercalcemia: observations on regulation of serum calcium. Calcif Tissue Int 1982; 34: 321–7
Thiebaud D, Portmann L, Jaeger P, et al. Oral versus intravenous AHPrBP (APD) in the treatment of hypercalcemia of malignancy. Bone 1986; 7(4): 247–53
Ziegler R, Scharla SH. Treatment of tumor hypercalcemia with clodronate. Recent Results Cancer Res 1989; 116: 47–53
Rastad J, Benson L, Johansson H, et al. Clodronate treatment in patients with malignancy-associated hypercalcemia. Acta Med Scand 1987; 221: 489–94
Percival RC, Paterson AD, Yates AJP, et al. Treatment of malignant hypercalcemia with clodronate. Br J Cancer 1985; 51: 665–9
Schiller JH, Rasmussen P, Benson AB, et al. Maintenance etidronate in the prevention of malignancy-associated hypercalcemia. Arch Intern Med 1987; 147: 963–6
Ebetino FH, Dansereau SM. Bisphosphonate antiresorptive structure-activity relationship. In: Bijvoet OLM, Fleisch HA, Canfield RE, et al., editors. Bisphosphonates on bones. Amsterdam: Elsevier, 1995: 139–53
Green JR, Müller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new potent, heterocyclic bisphosphonate compound. J Bone Miner Res 1994; 9: 745–51
Walls J, Ratcliffe WA, Howell A, et al. Response to intravenous bisphosphonate therapy in hypercalcaemic patients with and without bone metastases: the role of parathyroid hormone-related protein. Br J Cancer 1994; 70: 169–72
Gurney H, Grill V, Martin TJ. Parathyroid hormone-related protein and response to pamidronate in tumour-induced hypercalcemia. Lancet 1993; 341: 1611–3
Body JJ, Dumon JC, Thirion M, et al. Circulating PTHrP concentrations in tumor-induced hypercalcemia: influence on the response to bisphosphonate and changes after therapy. J Bone Miner Res 1993; 8: 701–6
Pecherstorfer M, Thiebaud D. Treatment of tumor-induced hypercalcemia with escalating doses of pamidronate (APD). Ann Oncol 1992; 3(8): 661–3
Thiébaud D, Jaeger P, Burckhardt P. Response to retreatment of malignant hypercalcemia with the bisphosphonate AHPrBP (APD): respective role of kidney and bone. J Bone Miner Res 1990; 5: 221–6
Pecherstorfer M, Steinhauser EU, Rizzoli R, et al. Efficacy and safety of ibandronate in the treatment of hypercalcemia of malignancy: a randomized multicentric comparison to pamidronate. Support Care Cancer. In press
Bounameaux HM, Schifferli J, Montani JP, et al. Renal failure associated with intravenous bisphosphonates [letter]. Lancet 1983; I: 471
Machado CE, Flombaum CD. Safety of pamidronate in patients with renal failure and hypercalcemia. Clin Nephrol 1996; 45(3): 175–9
Adami S, Zamberlan N. Adverse effects of bisphosphonates. Drug Saf 1996; 14(3): 158–70
Pecherstorfer M, Jilch R, Horn E, et al. Effect of first treatment with the aminobisphosphonatespamidronate and ibandronate on circulating lymphocyte subpopulations. J Bone Miner Res 2000, 154
Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med 1999; 340: 737–8
Kunzmann V, Bauer E, Feurle J, et al. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000; 96: 384–92
Sauty A, Pecherstorfer M, Zimmer-Roth I, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonate treatment in vitro and in patients with malignancy. Bone 1996; 18(2): 133–9
Schweitzer DH, Oostendorp-Van de Ruit M, Van der Pluijm G, et al. Interleukin-6 and the acute phase response during treatment of patients with Paget’s disease with the nitrogen-containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res 1995; 10(6): 956–62
Stewart GO, Stuckey BG, Ward LC, et al. Iritis following intravenous pamidronate. Aust N Z J Med 1996; 26(3): 414–5
Macarol V, Fraunfelder F. Pamidronate disodium and possible ocular adverse drug reactions. Am J Ophtalmol 1994; 118: 220–4
Pedersen-Bjergaard U, Myhre J. Severe hypocalcemia after treatment with diphosphonate and aminoglycoside [letter]. BMJ 1991; 302: 295
Pecherstorfer M, Schilling T, Janisch S, et al. Effect of clodronate treatment on bone scintigraphy in metastatic breast cancer. J Nucl Med 1993; 34: 1039–44
Warrell RP, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blind comparison to calcitonin. Ann Intern Med 1988; 108: 669–74
Bockman RS, Repo MA, Warrell RP, et al. Distribution of trace levels of therapeutic gallium in bone as mapped by synchroton x-ray microscopy. Proc Natl Acad Sci U S A 1990; 87(11): 4149–53
Bockman RS, Boskey AL, Blumenthal NC, et al. Gallium increases bone calcium and crystallite perfection of hydroxyapatite. Calcif Tissue Int 1986; 39(6): 376–81
Bockman RS, Guidon Jr PT, Pan LC, et al. Gallium nitrate increases type I collagen and fibronectin mRNA and collagen protein levels in bone and fibroblast cells. J Cell Biochem 1993; 52(4): 396–403
Kiang DT, Loken MK, Kennedy BJ. Mechanism of the hypocalcemic effect of mithramycin. J Clin Endocrinol Metab 1979; 48(2): 341–4
Slayton RE, Shnider BI, Elias E, et al. New approach to the treatment of hypercalcemia: the effect of short-term treatment with mithramycin. Clin Pharmacol Ther 1971; 12(5): 833–7
Elias EG, Evans JT. Hypercalcemic crisis in neoplastic disease: management with mithramycin. Surgery 1972; 71(4): 631–5
Gasser AB, Flury R, Senn HJ. Therapie des Hyperkalzämiesyndroms mit Mithramyzin. Schweiz Med Wochenschr 1974; 104: 1792–4
Fillastre JP, Maitrot J, Canonne MA, et al. Renal function and alterations in plasma electrolyte levels in normocalcemic and hypercalcemic patients with malignant diseases, given an intravenous infusion of mithramycin. Chemotherapy 1974; 20: 280–95
Green L, Donehower RC. Hepatic toxicity of low doses of mithramycin in hypercalcemia. Cancer Treat Rep 1984; 68(11): 1379–81
Silva OL, Becker KL. Salmon calcitonin in the treatment of hypercalcemia. Arch Intern Med 1973; 132: 337–9
Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther 1978; 24(2): 219–22
Geley S, Fiegl M, Hartmann BL, et al. Genes mediating glucocorticoid effects and mechanisms of their regulation. Rev Physiol Biochem Pharmacol 1996; 128: 1–97
Takahashi S, Goldring S, Katz M, et al. Downregulation of calcitonin receptor mRNA expression by calcitonin during human osteoclast-like differentiation. J Clin Invest 1995; 95(1): 167–71
Ralston SH, Alzaid AA, Gardner MD, et al. Treatment of cancer-associated hypercalcemia with combined aminohydroxypropylidene diphosphonate and calcitonin. BMJ 1986; 292: 1549–50
Bower M, Stein RC, Hedley A, et al. The use of nasal calcitonin spray in the treatment of hypercalcemia of malignancy. Cancer Chemother Pharmacol 1991; 28: 311–2
Leyland-Jones B. Pharmacokinetics and therapeutic index of gallium nitrate. Semin Oncol 1991; 18(4 Suppl. 5): 16–20
Ahr DJ, Scialla SJ, Kimball DB. Acquired platelet dysfunction following mithramycin therapy. Cancer 1978; 41: 448–54
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pecherstorfer, M., Brenner, K. & Zojer, N. Current Management Strategies for Hypercalcemia. Mol Diag Ther 2, 273–292 (2003). https://doi.org/10.2165/00024677-200302040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200302040-00005