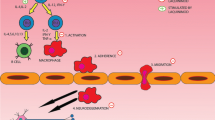
Abstract
The disease-modifying agents currently used in the treatment of multiple sclerosis (MS) are not completely effective and are associated with adverse effects and high costs. Thus, alternative treatment options are highly desirable. HMG-CoA reductase inhibitors (statins), widely prescribed as cholesterol-lowering agents, may be a future treatment option for MS — either in an add-on therapy regimen or alone — as they have been shown to exhibit potent immunomodulatory effects. Several recent reports have demonstrated that HMG-CoA reductase inhibitors prevent and reverse chronic and relapsing experimental autoimmune encephalomyelitis, an animal model of MS. Furthermore, in vitro experiments with human immune cells have shown an immunomodulatory mode of action of HMG-CoA reductase inhibitors that is comparable to that of interferon-β, an established treatment for MS. An open-label clinical trial assessing simvastatin treatment in patients with MS revealed a significant decrease in the number and volume of new lesions, as assessed using magnetic resonance imaging, and a favourable safety profile. A large multicentre, placebo-controlled phase II clinical trial assessing atorvastatin in patients with a clinically isolated syndrome (i.e. a single clinical event that is indicative of demyelination, and that predisposes to the development MS) has recently been initiated. However, prospective placebo-controlled trials of HMG-CoA reductase inhibitors in definite MS are difficult to perform because of ethical and financial issues. Furthermore, overly optimistic reports in the popular media, as well as the often uncontrolled access to HMG-CoA reductase inhibitors by patients with MS, complicate the evaluation of HMG-CoA reductase inhibitors as a realistic future treatment option for MS.


Similar content being viewed by others
References
Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Medical progress: multiple sclerosis. N Engl J Med 2000 Sep 28; 343(13): 938–52
Patwardhan MD, Matchar DB, Samsar GP, et al. Cost of multiple sclerosis by level of disability: a review of literature. Mult Scler 2005; 11(2): 232–9
Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 2002 Apr; 3(4): 291–301
Zamvil SS, Steinman L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron 2003 Jun 5; 38(5): 685–8
Lassmann H, Brück W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 2001 Mar; 7(3): 115–21
Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol 2001 Sep; 2(9): 762–4
Neuhaus O, Archelos JJ, Härtung HP. Immunomodulation in multiple sclerosis: from immunosuppression to neuroprotection. Trends Pharmacol Sci 2003 Mar; 24(3): 131–8
Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004 Apr; 55(4): 458–68
Trapp BD, Ransohoff RM, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol 1999 Jun; 12(3): 295–302
Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 2002 Oct; 25(10): 532–7
Fernandez O, Fernandez V, De Ramon E. Azathioprine and methotrexate in multiple sclerosis. J Neurol Sci 2004 Aug; 223(1): 29–34
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993 Apr; 43(4): 655–61
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in exacerbating-remitting multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996 Mar; 39(3): 285–94
PRISMS (Prevention of Relapses and Disability by Interferon beta-la Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998 Nov 7; 352(9139): 1498–504
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995 Jul; 45(7): 1268–76
Kieseier BC, Härtung HP. Current disease-modifying therapies in multiple sclerosis. Semin Neurol 2003 Jun; 23(2): 133–45
Härtung HP, Gonsette R, König N, et al. A placebo-controlled, double-blind, randomised, multicentre trial of mitoxantrone in progressive multiple sclerosis. Lancet 2002 Dec 21; 360(9350): 2018–25
Wiendl H, Kieseier BC. Disease-modifying therapies in multiple sclerosis: an update on recent and ongoing trials and future strategies. Expert Opin Investig Drugs 2003 Aug; 12(8): 689–712
Polman CH, Uitdehaag BMJ. New and emerging treatment options for multiple sclerosis. Lancet Neurol 2003 Sep; 2(9): 563–6
Davidson MH. Safety profiles for the HMG-CoA reductase inhibitors: treatment and trust. Drugs 2001; 61(2): 197–206
Gotto AMJ. Safety and statin therapy: reconsidering the risks and benefits. Arch Intern Med 2003 Mar 24; 163(6): 657–9
Wortmann RL. Lipid-lowering agents and myopathy. Curr Opin Rheumatol 2002 Nov; 14(6): 643–7
Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003 Apr 2; 289(13): 1681–90
Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002 Feb 14; 346(7): 539–40
Massy ZA, Guijarro C. Statins: effects beyond cholesterol lowering. Nephrol Dial Transplant 2001 Sep; 16(9): 1738–41
Larosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 1999 Dec; 282(24): 2340–6
Ridker PM. Connecting the role of C-reactive protein and statins in cardiovascular disease. Clin Cardiol 2003 Apr; 26(4 Suppl. 3): 11139–44
Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004 Jun; 109(23 Suppl. 1): III39–43
Chan KKW, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003 Jan; 9(1): 10–9
Jakobisiak M, Golab J. Potential antitumor effects of statins. Int J Oncol 2003 Oct; 23(4): 1055–69
Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet 2000 Nov 11; 356(9242): 1627–31
White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med 2000 Aug 3; 343(5): 317–26
Cutts JL, Scallen TJ, Watson J, et al. Role of mevalonic acid in the regulation of natural killer cell cytotoxicity. J Cell Physiol 1989 Jun; 139(3): 550–7
Kobashigawa JA, Katznelson S, Laks H, et al. Effects of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995 Sep 7; 333(10): 621–7
Kurakata S, Kada M, Shimada Y, et al. Effects of different inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, pravastatin sodium and simvastatin, on sterol synthesis and immunological functions in human lymphocytes in vitro. Immunopharmacology 1996 Aug; 34(1): 51–61
Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci 2002 Oct; 23(10): 482–6
Baker D, Adamson P, Greenwood J. Potential of statins for the treatment of multiple sclerosis. Lancet Neurol 2003 Jan; 2(1): 9–10
Neuhaus O, Stiive O, Zamvil SS, et al. Are statins a treatment option for multiple sclerosis? Lancet Neurol 2004 Jun; 3(6): 369–71
McCarey DW, McInnes IB, Madhok R, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004 Jun 19; 363(9426): 2015–21
Stanislaus R, Pahan K, Singh AK, et al. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett 1999 Jul; 269(2): 71–4
Youssef S, Stiive O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002 Nov 7; 420(6911): 78–84
Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med 2003 Mar 17; 197(6): 725–33
Greenwood J, Walters CE, Pryce G, et al. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J 2003 May; 17(8): 905–7
Nath N, Giri S, Prasad R, et al. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor for multiple sclerosis therapy. J Immunol 2004 Jan 15; 172(2): 1273–86
Neuhaus O, Strasser-Fuchs S, Fazekas F, et al. Statins as immunomodulators: comparison with interferon-betalb in MS. Neurology 2002 Oct 8; 59(7): 990–7
Matsumoto M, Einhaus D, Gold ES, et al. Simvastatin augments lipopolysaccharide-induced proinflammatory responses in macrophages by differential regulation of the c-Fos and c-Jun transcription factors. J Immunol 2004 Jun 15; 172(12): 7377–84
Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol 2001 Nov; 21(11): 1712–9
Singh R, Wang B, Shirvaikar A, et al. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J Clin Invest 1999 Jun; 103(11): 1561–70
Neuhaus O, Stiive O, Archelos JJ, et al. Putative mechanisms of action of statins in multiple sclerosis 0 comparison to interfer-on-beta and glatiramer acetate. J Neurol Sci 2005; 233(1-2): 173–7
Weitz-Schmidt G, Weizenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 2001 Jun; 7(6): 687–92
Archelos JJ, Hartung HP. The role of adhesion molecules in multiple sclerosis: biology, pathogenesis and therapeutic implications. Mol Med Today 1997 Jul; 3(7): 310–21
Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000 Dec; 6(12): 1399–402
Floris S, Blezer EL, Schreibelt G, et al. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain 2004 Mar; 127(3): 616–27
Botti RE, Triscari J, Pan HY, et al. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol 1991 Jun; 14(3): 256–61
Saheki A, Terasaki T, Tamai I, et al. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharmacol Res 1994 Feb; 11(2): 305–11
Sena A, Pedrosa R, Morais MG. Therapeutic potential of lovastatin in multiple sclerosis. J Neurol 2003 Jun; 250(6): 754–5
Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 2004 May 15; 363(9421): 1607–8
Daumer M, Kessner S, Lederer C, et al. Modelling placebo groups for clinical trials [abstract]. Mult Scier 2003 Sep; 9Suppl 1): S22
Pilcher HR. Statins may curb multiple sclerosis. Nature News Service: Macmillan Magazines Ltd, 2004 May 14
Muldoon MF, Flory JD, Marsland A, et al. Effects of lovastatin on the immune system. Am J Cardiol 1997 Nov 15; 80(10): 1391–4
Stüve O, Youssef S, Dunn S, et al. Atorvastatin enhances the clinically beneficial effects of glatiramer acetate in experimental autoimmune encephalomyelitis through induction of a TH2 phenotype [abstract]. Neurology 2004 Apr 13; 62(Suppl 5) (7): A438
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neuhaus, O., Stüve, O., Zamvil, S.S. et al. Evaluation of HMG-CoA Reductase Inhibitors for Multiple Sclerosis. CNS Drugs 19, 833–841 (2005). https://doi.org/10.2165/00023210-200519100-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200519100-00003