Abstract
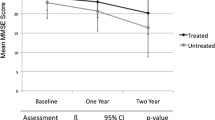
The most prevalent cause of dementia — Alzheimer’s disease — is characterised by an early cholinergic deficit that is in part responsible for the cognitive deficits, especially memory and attention defects, seen with this condition. Three cholinesterase inhibitors (ChEIs), namely donepezil, rivastigmine and galantamine, are widely used for the symptomatic treatment of patients with Alzheimer’s disease. Placebo-controlled, randomised clinical trials have shown significant effects of these drugs on global function, cognition, activities of daily living (ADL) and behavioural symptoms in patients with this disorder. These trials have been conducted for up to 12 months and were followed by open-label extension studies. One placebo-controlled, randomised clinical trial followed patients for up to 4 years. Both retrospective and prospective follow-up studies suggest a treatment effect for ChEIs that lasts for up to 5 years. Studies have shown comparable effects for ChEIs in patients with moderate-to-severe Alzheimer’s disease or mild Alzheimer’s disease. Clinically relevant responses consist not only of improvement over 3–6 months but also stabilisation and possibly slower than expected decline. Lack of overt clinical improvement in one domain (e.g. global function, cognition, ADL or behaviour) does not preclude clinically relevant benefit(s) in other domains.
If it is judged that the patient has experienced a treatment effect from ChEI therapy during the first 6 months, it is recommended that treatment be continued for at least 1 year before discontinuation is considered again. On average, patients will return to their pre-treatment status between 9 and 12 months of initiation of treatment. However, this return to pre-treatment level does not mean that the treatment effect has disappeared. At this point in time, the patient may still function better than he or she would have without treatment. Setting a fixed measurement, e.g. a Mini-Mental State Examination score, as a ‘when to stop treatment limit’ is not clinically rational. The length of treatment should depend on several individual patient factors. The earlier the diagnosis is made and the slower the rate of disease progression, the longer the treatment period will tend to be. Treatment duration must therefore be evaluated on an individual basis, and the patient’s status compared with what would have been expected without treatment. If a clinical evaluation is conducted with a view to stopping or switching treatment, it is crucial that all domains are evaluated and that the patient is evaluated at more than one point in time before the decision is made.





Similar content being viewed by others
References
Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56(9): 1143–53
Desmond DW, Moroney JT, Sano M, et al. Recovery of cognitive function after stroke. Stroke 1996 Oct; 27(10): 1798–803
Geula C. Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology 1998 Jul; 51(1 Suppl. 1): S18–29
Deutsch JA. The cholinergic synapse and the site of memory. Science 1971 Nov; 174(11): 788–94
Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976 Dec; II(8000): 1403
Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56(9): 1154–66
Wagstaff AJ, McTavish D. Tacrine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in Alzheimer’s disease. Drugs Aging 1994; 4: 510–40
Poirier J. Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl 2002; 127(127): 6–19
Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002; 41(10): 719–39
Maelicke A. Allosteric modulation of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Dement Geriatr Cogn Disord 2000 Sep; 11Suppl. 1: 11–8
Fisher A. Therapeutic strategies in Alzheimer’s disease: M1 muscarinic agonists. Jpn J Pharmacol 2000 Oct; 84(2): 101–12
Geerts H, Finkel L, Carr R, et al. Nicotinic receptor modulation: advantages for successful Alzheimer’s disease therapy. J Neural Transm Suppl 2002; (62): 203-16
Samochocki M, Hoffle A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 2003; 305(3): 1024–36
Giacobini E. Selective inhibitors of butyrylcholinesterase: a valid alternative for therapy of Alzheimer’s disease? Drugs Aging 2001; 18(12): 891–8
Wilcock G, Howe I, Coles H, et al. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s disease. Drugs Aging 2003; 20(10): 777–89
Gray R. Comparative study of donepezil and rivastigmine [letter]. Int J Clin Pract 2003 Jun; 57(5): 449
Grossberg G. Comparative study of donepezil and rivastigmine [letter]. Int J Clin Pract 2003 Jan; 57(1): 70–1
Nobili F, Koulibaly M, Vitali P, et al. Brain perfusion follow-up in Alzheimer’s patients during treatment with acetylcholinesterase inhibitors. J Nucl Med 2002; 43(8): 983–90
Bullock R, Erkinjuntti T, Käfferlcin W, et al. Greater improvement in cognition and activities of daily living with donepezil compared to galantamine in a direct head-to-head trial in Alzheimer patients [abstract]. Eur J Neurol 2002; 9Suppl. 2: 197–8
Thomas RG, Berg JD, Sano M, et al. Analysis of longitudinal data in an Alzheimer’s disease clinical trial. Stat Med 2000 Jun; 19(11–12): 1433–40
Siddiqui O, Ali MW. A comparison of the random-effects pattern mixture model with last-observation-carried-forward (LOCF) analysis in longitudinal clinical trials with dropouts. J Biopharm Stat 1998 Nov; 8(4): 545–63
Winblad B, Wimo A, Almkvist O. Outcome measures in Alzheimer’s disease: do they go far enough? Dement Geriatr Cogn Disord 2000; 11 Suppl. 1: 3–10
Winblad B, Brodaty H, Gauthier S, et al. Pharmacotherapy of Alzheimer’s disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry 2001; 16(7): 653–66
Burns A, Lawlor B, Craig S. Assessment scales in old age psychiatry. London: Martin Dunitz Ltd, 1999
Knopman DS, Knapp MJ, Gracon SI, et al. The clinician interview-based impression (CIBI): a clinician’s global change of rating scale in Alzheimer’s disease. Neurology 1994; 44: 2315–21
Folstein MF, Folstein SE, McHugh PR. ’Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141: 1356–64
Gelinas I, Gauthier L, McIntyre M, et al. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 1999 Sep; 53(5): 471–81
DeJong R, Osterlund OW, Roy GW. Measurement of quality-of-life changes in patients with Alzheimer’s disease. Clin Ther 1989 Jul; 11(4): 545–54
Cummings JL, Mega MS, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–14
Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global functioning in Alzheimer’s disease. Arch Intern Med 1998; 158: 1021–31
Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998; 50: 136–45
Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer’s disease: results from a multinational trial. Dement Geriatr Cogn Disord 1999; 10: 237–44
Rösier M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999; 318: 633–40
Corey-Bloom J, Anand R, Veach J, et al. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriat Psychopharmacol 1998; 1: 55–65
Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 2000; 321(7274): 1445–9
Raskind MA, Peskind ER, Wessel T, et al. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000; 54(12): 2261–8
Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 2000; 54(12): 2269–76
Leber P. Criteria used by drug regulatory authorities. In: Qizilbash N, Schneider LS, Chui H, et al., editors. Evidence-based dementia practice. Oxford: Blackwell Science Ltd, 2002: 376–87
Lanctot KL, Herrmann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003 Sep; 169(6): 557–64
Wynn ZJ, Cummings JL. Cholinesterase inhibitor therapies and neuropsychiatric manifestations of Alzheimer’s disease. Dement Geriatr Cogn Disord 2004; 17(1–2): 100–8
Cummings JL, Schneider L, Tariot PN, et al. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry 2004 Mar; 161(3): 532–8
Dunn NR, Pearce GL, Shakir SA. Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol 2000; 14(4): 406–8
McGirr G, Compton SA. Drugs for dementia: the first year. An audit of prescribing practice. Ulster Med J 2000 Nov; 69(2): 123–7
Aupperle PM, MacPhee ER, Coyne AC, et al. Health service utilization by Alzheimer’s disease patients: a 2-year follow-up of primary versus subspecialty care. J Geriatr Psychiatry Neurol 2003 Mar; 16(1): 15–7
Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001; 57(3): 489–95
Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001; 57(3): 481–8
AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363: 2105–15
Schneider LS. AD2000; donepezil in Alzheimer’s disease. Lancet 2004; 363: 2100–1
Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry 1994; 151: 390–6
Mendiondo MS, Ashford JW, Kryscio RJ, et al. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med 2000 Jun; 19(11–12): 1607–16
Thal LJ, Grundman M, Berg J, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology 2003 Dec; 61(11): 1498–502
Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 2001; 58(3): 427–33
Beier MT. Long-term benefits of galantamine in mild-to-moderate Alzheimer’s disease. Clin Geriatr 2003; 11: 16–24
Farlow M, Anand R, Messina JJ, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000; 44(4): 236–41
Doody RS, Dunn JK, Clark CM, et al. Chronic donepezil treatment is associated with slowed cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord 2001; 12(4): 295–300
Doraiswamy PM, Krishnan KR, Anand R, et al. Long-term effects of rivastigmine in moderately severe Alzheimer’s disease: does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry 2002 May; 26(4): 705–12
Marcusson J, Bullock R, Gauthier S, et al. Galantamine demonstrates efficacy and safety in elderly patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2003 Jul; 17(3): S86–91
Blesa R, Davidson M, Kurz A, et al. Galantamine provides sustained benefits in patients with’ advanced moderate’ Alzheimer’s disease for at least 12 months. Dement Geriatr Cogn Disord 2003; 15(2): 79–87
Rogers SL, Doody RS, Pratt RD, et al. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol 2000 May; 10(3): 195–203
Small G, Mendiondo MS, Quarg P, et al. Efficacy of rivastigmine treatment in Alzheimer. 42nd American College of Neuropsychopharmacology Annual Meeting; 2003 Dec 7–11, San Juan
Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc 2003 Jul; 51(7): 937–44
Feldman H, Gauthier S, Hecker J, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease [published erratum appears in Neurology 2001 Dec; 57(11): 2153]. Neurology 2001; 57(4): 613–20
Wilkinson DG, Hock C, Farlow M, et al. Galantamine provides broad benefits in patients with ‘advanced moderate’ Alzheimer’s disease (MMSE < or = 12) for up to six months. Int J Clin Pract 2002 Sep; 56(7): 509–14
Johannsen P, Barcikowska M, Heun R, et al. Donepezil-treated Alzheimer’s disease patients with apparent initial cognitive decline demonstrate significant benefits when therapy is continued: results from a randomized, placebo-controlled trial [abstract]. J Neurol 2003; 250Suppl. 2: 126
Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology 2001; 57(6): 957–64
Tschampa HJ, Neumann M, Zerr I, et al. Patients with Alzheimer’s disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 2001; 71(1): 33–9
Giacobini E. Cholinesterase inhibitors do more than inhibit cholinesterase. In: Becker R, Giacobini E, editors. Alzheimer’s disease: from molecular biology to therapy. Boston (MA): Birkhäuser, 1996: 187–204
Becker E, Giacobini E. Mechanisms of cholinesterase inhibition in senile dementia of the Alzheimer type. Drug Dev Res 1998; 12: 163–95
Becker R, Giacobini E. Pharmacodynamics of acetylcholinesterase inhibition. Drug Dev Res 1998; 14: 235–46
von der Kammer H, Mayhaus M, Albrecht C, et al. Muscarinic acetylcholine receptors activate expression of the EGR gene family of transcription factors. J Biol Chem 1998 Jun; 273(23): 14538–44
Mattio T, McIlhany M, Giacobini E, et al. The effects of physostigmine on acetylcholinesterase activity of CSF plasma and brain: a comparison of intravenous and intraventricular administration in beagle dogs. Neuropharmacology 1986 Oct; 25(10): 1167–77
Singh S, Dudley C. Discontinuation syndrome following donepezil cessation. Int J Geriatr Psychiatry 2003 Apr; 18(4): 282–4
Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004 Jan; 291(3): 317–24
Acknowledgements
Dr Johannsen has been engaged as a consultant for Pfizer, Novartis and Lundbeck, and has received honoraria for lectures from Pfizer, Lundbeck, Novartis, Janssen-Cilag and GSK. No funding was used to assist in the preparation of this review. The author was the principal investigator of the AWARE study[65] cited in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johannsen, P. Long-Term Cholinesterase Inhibitor Treatment of Alzheimer’s Disease. CNS Drugs 18, 757–768 (2004). https://doi.org/10.2165/00023210-200418120-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200418120-00001