Abstract
Introduction: Many patients with migraine do not consult a physician, or do not achieve adequate relief after consulting a physician because of undertreatment. The objective of the Migraine and Zomig1. Evaluation study was to provide insights into the management of migraine in the general population.
Methods: In phase I, 5553 members of the general public in the UK, France, Germany, Italy, and the US were interviewed by telephone and classified according to International Headache Society criteria. The Migraine Disability Assessment Scale (MIDAS) questionnaire was used to assess the impact of migraine on work, home and social lives. In phase II, 516 patients with clinically diagnosed migraine were interviewed to assess the impact of migraine on daily life, attitudes towards migraine, perceptions of current treatments and aspirations for future treatments.
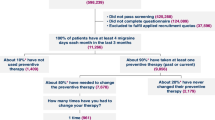
Results: In phase I, the average prevalence of migraine over the five countries was 9%. Migraine posed a significant burden in terms of the impact on patients’ daily lives, and attack severity and frequency. However, medical consultation rates were low; reasons for this included patients not recognising that they had migraine or having low expectations about treatment benefits. On average, only 10% of patients who had consulted a physician had been prescribed a triptan. Only 22% of participants in phase II thought that migraine did not markedly affect their lives. In each of the five countries, ≥ 50% of patients required bed rest to manage migraine attacks, demonstrating the impact of migraine-related disability on patients’ lives. Assessment of MIDAS scores confirmed the debilitating effect of migraine; > 50% of respondents had a MIDAS grade of III or IV, indicating moderate or severe disability. Less than one-third of patients reported that their current medication was consistently effective and only 36% were ‘very satisfied’ with their current therapy. High efficacy and rapid pain relief were rated as the most important attributes of migraine medications. When asked which formulation they would like to see more of, most patients chose a tablet that dissolves in the mouth without the need to take liquids.
Conclusions: These results show that migraine patients worldwide are still not receiving adequate treatment and there remains a significant unmet need in migraine care. The challenge for the future is to diagnose migraine early and offer patients effective migraine-specific therapies. Physicians particularly need to reach patients who do not realise they have migraine and those who have lapsed from care.





Similar content being viewed by others
References
Terwindt GM, Ferrari MD, Tijhuis M, et al. The impact of migraine on quality of life in the general population: the GEM study. Neurology 2000; 55: 624–9
Dowson A, Jagger S. The UK migraine patient survey: quality of life and treatment. Curr Med Res Opin 1999; 15: 241–53
Lipton RB, Hamelsky SW, Kolodner KB, et al. Migraine, quality of life, and depression. A population-based case-control study. Neurology 2000; 55: 629–35
Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998; 13: 667–76
Stang PE, Osterhaus J. Impact of migraine in the United States: data from the National Health Interview Survey. Headache 1993; 33: 29–35
Lipton RB, Stewart WF, Simon D. Medical consultation for migraine: results from the American Migraine Study. Headache 1998; 38: 87–96
Lipton RB, Stewart WF, Celentano D, et al. Underdiagnosed migraine headaches: a comparison of symptom-based and reported physician diagnosis. Arch Intern Med 1992; 152: 1273–8
Richard A, Massiou H, Herrmann MA. Frequency and profile of migraine patients seen by general practitioners. Semin Hop Paris 1999; 75: 440–6
Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy. Headache 1999; 39Suppl. 2: 20–6
Rapoport AM, Ramadan NM, Adelman JU, et al. Optimizing the dose of zolmitriptan (Zomig, 311C90) for the acute treatment of migraine. A multicenter, double-blind, placebo-controlled, dose range-finding study. The 017 Clinical Trial Study Group. Neurology 1997; 49: 1210–8
Solomon GD, Cady RK, Klapper JA, et al. Clinical efficacy and tolerability of 2.5 mg zolmitriptan for the acute treatment of migraine. The 042 Clinical Trial Study Group. Neurology 1997; 49: 1219–25
Schoenen J, Sawyer J. Zolmitriptan (Zomig™, 311C90), a novel dual central and peripheral 5HT1B/1D agonist: an overview of efficacy. Cephalalgia 1997; 17Suppl. 18: 28–40
Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1998; 8Suppl. 7: 1–96
Göbel H. Paper-pencil tests for retrospective and prospective evaluation of primary headaches on the basis of the IHS criteria. Headache 1994; 34: 564–8
Stewart WF, Lipton RB, Kolodner K, et al. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999; 19: 107–14
Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. JAMA 1992; 267: 64–9
Rasmussen BK. Epidemiology of migraine. Cephalalgia 1995; 15: 45–68
Henry P, Michel P, Brochet B, et al. A nationwide survey of migraine in France: prevalence and clinical features in adults. Cephalalgia 1992; 12: 229–37
Winnen J. Prevalence of adult migraine in general practice. Cephalalgia 1992; 12: 300–3
Pryse-Phillips W, Findlay H, Tugwell P, et al. A Canadian population survey on the clinical, epidemiologic and societal impact of migraine and tension-type headache. Can J Neurol Sci 1992; 19: 333–9
Goadsby PJ. Emerging oral triptan therapies. Headache 1999; 39Suppl. 2: S40–8
Tfelt-Hansen P, De Vries P, Saxena PR, et al. Triptans in migraine. A comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000; 60: 1259–87
Lipton RB, Stewart WF, Stone AM, et al. Stratified care vs step care strategies for migraine. The Disability in Strategies of Care (DISC) study: a randomized trial. JAMA 2000; 284: 2599–605
Acknowledgements
This study was supported by AstraZeneca. Dr J.L. Brandes serves as a consultant and is on a speaker bureau for AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tradenames are used for identification purposes only and do not imply endorsement.
Rights and permissions
About this article
Cite this article
Brandes, J.L. Global Trends in Migraine Care. CNS Drugs 16 (Suppl 1), 13–18 (2002). https://doi.org/10.2165/00023210-200216001-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200216001-00003