Summary
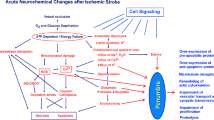
Programmed cell death (PCD) is the process by which redundant neurons delete themselves during embryonic development in the nervous system. Recent data indicate that a cellular suicide process similar to PCD also contributes to the death of neurons during stroke. This suggests that pharmacological approaches that interrupt PCD may have utility in stroke. Three of these approaches are neurotrophins, endonuclease inhibitors and calpain inhibitors.
Each of these strategies has been demonstrated to inhibit PCD in different in vitro biological systems. They have also proven effective when administered to animals in which a stroke has been surgically induced. Thus, the strategy of inhibiting PCD to diminish damage during ischaemia appears to have merit, and is likely to continue to be a growing area of research that may lead to new and novel therapeutic approaches in acute and chronic neurodegenerative disorders.
Similar content being viewed by others
References
Shigeno T, Mima T, Takakura K, et al. Amelioration of delayed neuronal death in the hippocampus by nerve growth factor. J Neurosci 1991; 11: 2914–9
Goto K, Ishige A, Sekiguchi K, et al. Effects of cycloheximide on delayed neuronal death in rat hippocampus. Brain Res 1990; 534: 299–302
Heron A, Pollard H, Dessi F, et al. Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem 1993; 61: 1973–6
Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke 1993; 24: 2002–9
Okamoto M, Matsumoto M, Ohtsuki T, et al. Internucleosomal DNA cleavage involved in ischemia-induced neuronal death. Biochem Biophys Res Commun 1993; 196: 1356–62
MacManus JP, Buchan AM, Hill IE, et al. Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett 1993; 164: 89–92
MacManus JP, Hill IE, Huang Z-G, et al. DNA damage consistent with apoptosis in transient focal ischemic neocortex. Neuroreport 1994; 5: 493–6
Oppenheim RW. Cell death during development of the nervous system. Ann Rev Neurosci 1991; 14: 453–501
Johnson Jr EM, Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annu Rev Neurosci 1993; 16: 31–46
Raff MC, Barres BA, Burne JF, et al. Programmed cell death and the control of cell survival: lessons from the nervous system. Science 1993; 262: 695–700
Wyllie AH, Kerr JFH, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytology 1980; 68: 251–306
Schwartz LM, Smith SW, Jones MEE, et al. Do all programmed cell deaths occur via apoptosis?. Proc Natl Acad Sci USA 1993; 90: 980–4
Deshpande J, Bergstedt K, Linden T, et al. Ultrastructural changes in the hippocampal CA1 region following transient cerebral ischemia: evidence against programmed cell death. Exp Brain Res 1992; 88: 91–105
Martin DP, Schmidt RE, DiStefano PS, et al. Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol 1988; 106: 829–44
Deckworth TL, Johnson EM. Temporal events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol 1993; 123: 1207–22
Gougeon M-L, Montagnier L. Apoptosis in AIDS. Science 1993; 260: 1269–70
Mobley WC, Rutkowski JL, Tennekoon GI, et al. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain cholinergic neurons. Mol Brain Res 1986; 1: 56–62
Fischer W, Bjorklund A, Chen K, et al. NGF improves spatial memory in aged rodents as a function of their age. J Neurosci 1991; 11: 1889–906
Barinaga M. Neurotrophic factors enter the clinic. Science 1994; 264: 772–4
Yamamoto S, Yoshimine T, Fujita T, et al. Protective effect of NGF atelocollagen mini-pellet on the hippocampal delayed neuronal death in gerbils. Neurosci Lett 1992; 141: 161–5
Mattson MP, Cheng B, Smith-Swintosky VL. Mechanisms of neurotrophic factor protection against calcium- and free radical-mediated excitotoxic injury: implications for treating neurodegenerative disorders. Exp Neurol 1993; 124: 89–95
Rabizadeh S, Oh J, Zhong L, et al. Induction of apoptosis by the low affinity NGF receptor. Science 1993; 261: 345–8
Friden PM, Walus LR, Watson P, et al. Blood-brain barrier penetration and in vivo activity of an NGF conjugate. Science 1993; 259: 373–7
Olson L. NGF and the treatment of Alzheimer’s disease. Exp Neurol 1993; 124: 5–15
Tuszynski MH, Uh S, Amarai DG, et al. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci 1990; 10: 3604–14
Anderson KJ, Dam D, Lee S, et al. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature 1988; 332: 360–1
Cheng B, Mattson MP. NGF and bFGF protect rat hippocampal and human cortical neurons against hypoglycemia damage by stabilizing calcium homeostasis. Neuron 1991; 7: 1031–41
Nozaki K, Finklestein SP, Beal MF. Basic fibroblast growth factor protects against hypoxia-ischemia and NMDA neurotoxicity in neonatal rats. J Cereb Blood Flow Metab 1993; 13: 221–8
Pittman RN, Wang S, DiBenedetto AJ, et al. A system for characterizing cellular and molecular events in programmed neuronal cell death. J Neurosci 1993; 13: 3669–80
Oppenheim RW, Prevette D, Fuller F. The lack of effect of acidic and basic fibroblast growth factor on the naturally occurring death of neurons in the chick embryo. J Neurosci 1992; 12: 2726–34
Freese A, Finklestein SP, DiFiglia M. Basic fibroblast growth factor protects striatal neurons in vitro from NMDA-receptor mediated excitotoxicity. Brain Res 1992; 575: 351–5
Nozaki K, Finklestein SP, Beal MF. Delayed administration of basic fibroblast growth factor protects against N-methyl-D-aspartate neurotoxicity in neonatal rats. Eur J Pharmacol 1993; 232: 295–7
Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980; 284: 555–6
Tominaga T, Kure S, Narisawa K, et al. Endonuclease activation following focal ischemic injury in the rat brain. Brain Res 1993; 608: 21–6
McConkey DJ, Hartzeil P, Nicotera P, et al. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J 1989; 3: 1843–9
Batistatou A, Greene LA. Aurintricarboxylic acid rescues PC 12 cells and sympathetic neurons from cell death caused by growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol 1991; 115: 461–72
Samples SD, Dubinsky JM. Aurintricarboxylic acid protects hippocampal neurons from glutamate excitotoxicity in vitro. J Neurochem 1993; 61: 382–5
Roberts-Lewis JM, Marcy VR, Zhao Y, et al. Aurintricarboxylic acid protects hippocampal neurons from NMDA- and ischemia-induced toxicity in vivo. J Neurochem 1993; 61: 378–81
Wang P, Kozlowski J, Cushman M. Isolation and structure elucidation of low molecular weight components of aurintricarboxylic acid. J Org Chem 1992; 57: 3861–6
Zeevalk GD, Schoepp D, Nicklas WJ. Aurintricarboxylic acid prevents NMDA-mediated excitotoxicity: evidence for its action as an NMDA receptor antagonist. J Neurochem 1993; 61: 386–9
Batistatou A, Greene LA. Internucleosomal DNA cleavage and neuronal cell survival/death. J Cell Biol 1993; 122: 523–32
Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1988; 1: 279–87
Lee KS, Frank S, Vanderklish P, et al. Inhibition of proteolysis protects hippocampal neurons from ischemia. Proc Natl Acad Sci USA 1991; 88: 7233–7
Sarin A, Adams DH, Henkart PA. Protease inhibitors selectively block T cell receptor-mediated programmed cell death in a murine T cell hybridoma and activated peripheral T cells. J Exp Med 1993; 178: 1693–700
Squier MKT, Miller ACK, Malkinson AM, et al. Calpain activation in apoptosis. J Cell Physiol 1994; 159: 229–37
Sarin A, Clerici M, Blatt SP, et al. Inhibition of activationinduced programmed cell death and restoration of defective immune responses of HIV+ donors by cysteine protease inhibitors. J Immunol 1994; 153: 862–72
Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neuroscience 1989; 9: 1579–90
Arlinghaus L, Mehdi S, Lee KS. Improved posthypoxic recovery with a membrane-permeable calpain inhibitor. Eur J Pharmacol 1991; 209: 123–5
Hiramatsu K, Kassell NF, Lee KS. Improved posthypoxic recovery of synaptic transmission in gerbil neocortical slices treated with a calpain inhibitor. Stroke 1993; 24: 1725–8
Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biol Sci 1991; 16: 150–3
Wang KKW. Developing selective inhibitors of calpain. Trends Pharmacol Sci 1991; 11: 139–42
Harbeson SL, Abelleira SM, Akiyama A, et al. Stereospecific synthesis of peptidyl α-keto amides as inhibitors of calpain. J Med Chem 1994; 37: 2918–29
Rami A, Krieglstein J. Protective effects of calpain inhibitors against neuronal damage caused by cytotoxic hypoxia in vitroand ischemia in vivo. Brain Res 1993; 609: 67–70
Hong S-C, Goto Y, Lanzino G, et al. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke 1994; 25: 663–9
Bartus RT, Hayward NJ, Elliott PJ, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Stroke 1994; 25: 2265–70
Bartus RT, Baker KL, Heiser AD, et al. Postischemic administration of AK275, a calpain inhibitor, provides substantial protection against focal ischemic brain damage. J Cereb Blood Flow Metab 1994; 14: 537–44
Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegansgene ced-9 protects cells from programmed cell death. Nature 1992; 356: 494–9
Allsopp TE, Wyatt S, Paterson HF, et al. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell 1993; 73: 295–307
Behl C, Hovey III L, Krajewski S, et al. Bcl-2 prevents killing of neuronal cells by glutamate but not by amyloid beta protein. Biochem Biophys Res Commun 1993; 197: 949–56
Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124: 1–6
Linnik MD, Zahos P, Geschwind MD, et al. Expression of bcl-2 from a defective herpes simplex virus 1 vector limits neuronal death in stroke [abstract]. Soc Neurosci Abst 1994; 20: 1479
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Linnik, M.D. Programmed Cell Death in Cerebral Ischaemia. CNS Drugs 3, 239–244 (1995). https://doi.org/10.2165/00023210-199503040-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-199503040-00001