Abstract
Background: Schizophrenia is a devastating and costly illness that affects 1% of the population in the US. Effective pharmacological therapies are available but suboptimal patient adherence to either acute or long-term therapeutic regimens reduces their effectiveness. The availability of a long-acting injection (LAI) formulation of risperidone may increase adherence and improve clinical and economic outcomes for people with schizophrenia.
Objective: To assess the cost effectiveness of risperidone LAI compared with oral risperidone, oral olanzapine and haloperidol decanoate LAI over a 1-year time period in outpatients with schizophrenia who had previously suffered a relapse requiring hospitalisation.
Perspective: US healthcare system.
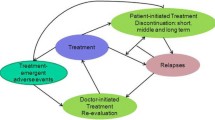
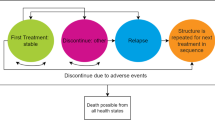
Methods: Published medical literature, unpublished data from clinical trials and a consumer health database, and a clinical expert panel were used to populate a decision-analysis model comparing the four treatment alternatives. The model captured: rates of patient compliance; rates, frequency and duration of relapse; incidence of adverse events (bodyweight gain and extrapyramidal effects); and healthcare resource utilisation and associated costs. Primary outcomes were: the proportion of patients with relapse; the frequency of relapse per patient; the number of relapse days per patient; and total direct medical cost per patient per year. Costs are in year 2002 US dollars.
Results: Based on model projections, the proportions of patients experiencing a relapse requiring hospitalisation after 1 year of treatment were 66% for haloperidol decanoate LAI, 41% for oral risperidone and oral olanzapine and 26% for risperidone LAI, while the proportion of patients with a relapse not requiring hospitalisation were 60%, 37%, 37% and 24%, respectively. The mean number of days of relapse requiring hospitalisation per patient per year was 28 for haloperidol decanoate LAI, 18 for oral risperidone and oral olanzapine and 11 for risperidone LAI, while the mean number of days of relapse not requiring hospitalisation was 8, 5, 5 and 3, respectively. This would translate into direct medical cost savings with risperidone LAI compared with oral risperidone, oral olanzapine and haloperidol decanoate LAI of $US397, $US1742, and $US8328, respectively. These findings were supported by sensitivity analyses.
Conclusion: The use of risperidone LAI for treatment of outpatients with schizophrenia is predicted in this model to result in better clinical outcomes and lower total healthcare costs over 1 year than its comparators, oral risperidone, oral olanzapine and haloperidol decanoate LAI. Risperidone LAI may therefore be a cost saving therapeutic option for outpatients with schizophrenia in the US healthcare setting.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Kendler KS, Gallagher TJ, Abelson JM, et al. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample: the National Comorbidity Survey. Arch Gen Psychiatry 1996 Nov; 53 (11): 1022–31
Wyatt RJ, Henter J, Leary MC, et al. An economic evaluation of schizophrenia... 1991. Soc Psychiatry Pyschiatr Epidemiol 1995; 30 Suppl. 5: 196–205
Kelly GR, Scott JE, Mamon J. Medication compliance and health education among outpatients with chronic mental disorders. Med Care 1990 Dec; 28 (12): 1181–97
Barnes TR. Depot antipsychotic drugs and prevention of psychotic relapse. Clin Neuropharmacol 1991; 14 Suppl. 2: S1–6
Duncan JC, Rogers R. Medication compliance in patients with chronic schizophrenia: implications for the community management of mentally disordered offenders. J Forensic Sci 1998 Nov; 46 (3): 1133–7
Scottish Schizophrenia Research Group. The Scottish First Episode Schizophrenia Study, II: treatment: pimozide versus flupenthixol. Br J Psychiatry 1987 Mar; 150: 334–8
Young JL, Zonana HV, Shepler L. Medication noncompliance in schizophrenia: codification and update. Bull Am Acad Psychiatry Law 1986; 14 5(2): 105–22
Buchanan A. A two-year prospective study of treatment compliance in patients with schizophrenia. Psychol Med 1992 Aug; 22 (3): 787–97
Kissling W. Compliance, quality assurance and standards for relapse prevention in schizophrenia. Acta Psychiatr Scand 1997; 89 Suppl. 382: 16–24
Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995; 21 (3): 419–29
Davis JM, Kane JM, Marder SR, et al.J Dose response of prophylactic antipsychotics. J Clin Psychiatry 1993 Mar; Suppl. 54: 24–30
Davis JM, Matalon L, Watanabe MD, et al. Depot antipsychotic drugs: place in therapy. Drugs 1994 May; 47 (5): 741–73
Glazer WM, Kane JM. Depot neuroleptic therapy: an underutilized treatment option. J Clin Psychiatry 1992 Dec; 53 (12): 426–33
Weiden PJ, Dixon L, Frances A, et al. Neuroleptic noncompliance in schizophrenia. In: Tamming CA, Schulz SC, editors. Advances in neuropsychiatry and psychopharmacology:schiz ophrenia research. Vol. 1. New York: Raven Press, 1991: 285–96
Johnson DA, Pasterski G, Ludlow JM, et al. The discontinuance of maintenance neuroleptic therapy in chronic schizophrenic patients: drug and social consequences. Acta Psychiatr Scand 1983 May; 67 (5): 339–52
Yousef HA. A five-year follow-up study of chronic schizophrenics and other psychotics treated in the community: depot haloperidol decanoate versus other neuroleptics. Adv Ther 1989; 67: 186–95
Ereshefsky L, Saklad SR, Jann MW, et al. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry 1984 May; 45 (5 Pt 2): 50–9
Hogarty GE, Schooler NR, Ulrich R, et al. Fluphenazine and social therapy in the aftercare of schizophrenic patients: relapse analyses of a wo-year controlled study of fiuphenazine decanoate and fluphenazine hydrochloride. Arch Gen Psychiatry 1979 Nov; 36 (12): 1283–94
Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia: II. Two-year effects of a controlled study on relapse and adjustment. Environmental-Personal Indicators in the Course of Schizophrenia (EPICS) Research Group. Arch Gen Psychiatry 1991 Apr; 48 (4): 340–7
American Psychiatric Association. Practice guidelines for the treatment of patients with schizophrenia. Am J Psychiatry 1997 Apr; 154 Suppl. 4: 1–63
Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull 1998; 24 (1): 1–10
Miller AL, Chiles JA, Chiles JK, et al. The Texas Medication Algorithm Project (TMAP) schizophrenia algorithms. J Clin Psychiatry 1999 Oct; 60 (10): 649–57
Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first longacting atypical antipsychotic. Am J Psychiatry 2003; 160 (6: 1125–32
Fleishhacker WW, Eerdeckens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003 Oct; 64 (10): 1250–7
Glazer WM, Ereshefsky L. A pharmacoeconomic model of outpatient antipsychotic therapy in “revolving door” schizophrenic patients. J Clin Psychiatry 1996 Aug; 57 (8): 337–45
Consumer Health Sciences (CHS) database [online]. Available from URL: http://www.chsinternational.com/diseases/ 05 Schiz_prod_Description 012401.asp [Accessed 2003 Jul 1]
Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000 Feb; 51 (2): 216–22
Diaz E, Levine HB, Sullivan MC, et al. Use of the medication event monitoring system to estimate medication compliance in patients with schizophrenia. J Psychiatry Neurosci 2001 Sep; 26 (4): 325–9
Weiden P, Rapkin B, Zygmunt A, et al. Postdischarge medication compliance of inpatients converted from an oral to a depot neuroleptic regimen. Psychiatr Serv 1995 Oct; 46 (10): 104954
Mahmoud RA, Engelhart LM, Ollendorf D, et al. The Risperidone Outcomes Study of Effectiveness (ROSE): a model for evaluating treatment strategies in typical psychiatric practice. J Clin Psychiatry 1999; 60 Suppl. 3: 42–7
Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994 Jun; 151 (6: 825–35
Carter C, Stevens M, Durkin M. Effects of risperidone therapy on the use of mental health care resources in Salt Lake County, Utah. Clin Ther 1998 Mar-Apr; 20 (2): 352–63
Coley KC, Carter CS, DaPos SV, et al. Effectiveness of antipsychotic therapy in a naturalistic setting: a comparison between risperidone, perphenazine and haloperidol. J Clin Psychiatry 1999 Dec; 60 (12): 850–6
Svarstad BL, Shireman TI, Sweeny JK. Using drug claims data to assess the relationship of medication adherence with hospitalisation and costs. Psychiatr Serv 2001 Jun; 52 (6): 805–11
Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001 May; 158 (5): 765–74
Vera-Llonch M, Delea T, Richardson E, et al. Outcomes and costs of risperidone versus olanzapine in patients with chronic schizophrenia or schizoaffective disorders: a Markov Model. Value Health 2004 Sep-Oct; 7 (5): 569–84
Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Risperidone Study Group. Br J Psychiatry 1995 Jun; 166 (6): 712–26
Min SK, Rhee CS, Kim CE, et al. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: a parallel-group double-blind comparative trial. Yonsei Med J 1993 Jun; 34 (2): 179–90
Chouinard G, Jones B, Remington G, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 1993 Feb; 13 (1): 25–40
Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002 Jan; 346 (1): 16–22
Beasley CM. Safety of olanzapine. J Clin Psychiatry Monograph 1997; 15 (2): 16–21
Vrijens B, Gross R, Goetghebeur E, et al. Dose timing information improves clinical explanatory power of data on patient compliance with antiretroviral drug regimens [abstract]. Society for Risk Analysis Annual Meeting; 2002 Dec 8–11; New Orleans
Chuff MA, Deer M, Bennett SJ, et al. Association between adherence to diuretic therapy and health care utilization in patients with heart failure. Pharmacotherapy 2003 Mar; 23 (3): 326–32
Weiden PJ, Mott T, Curico N. Recognition and management of neuroleptic non-compliance. In: Shiriqui C, Nasrallah H, editors. Contemporary issues in the treatment of schizophrenia. Washington, DC: American Psychiatric Press, 1995
NHS Centre for Reviews and Dissemination, Centre for Health Economics, University of York. A rapid and systematic review of atypical antipsychotics in schizophrenia [online]. Available from URL: http://www.NICE.org [Accessed 2001 Sep 21]
Marder SR, Glynn SM, Wirshing WC, et al. Maintenance treatment of schizophrenia with risperidone or Haloperidol: 2-year outcomes. Am J Psychiatry 2003 Aug; 160 (8): 1405–12
Hunter RH, Joy CB, Kennedy E, et al. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev 2003; (2): CD000440
Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA 2003 Nov; 290 (20): 2693–702
Duggan L, Fenton M, Dardennes RM, et al. Olanzapine for schizophrenia. Cochrane Database Syst Rev 2004; (2): AB001359
US Department of Labor Bureau of Labor Statistics Medical Care Services Consumer Price Indices from 1993 to 2002 [online]. Available from URL: http://www.bls.gov/ppi/ppinaics.htm [Accessed 2003 Jul 1]
Hamilton SH, Revicki DA, Edgell ET, et al. Clinical and economic outcomes of olanzapine compared with haloperidolfor schizophrenia: results from a randomized clinical trial. Pharmacoeconomics 1999 May; 15 (5): 469–80
Data on file, Janssen Medical Affairs, L.L.C., 2002
Palmer CS, Revicki DA, Genduso LA, et al. A cost-effectiveness clinical decision analysis model for schizophrenia. Am J Managed Care 1998 Mar; 4 (3): 345–55
Spielman AB, Kanders B, Kienholz M, et al. The cost of losing: an analysis of commercial weight-loss programs in a metropolitan area. J Am College Nutr 1992 Feb; 11 (1): 36–41
Data on file, Janssen Medical Affairs, L.L.C., 2002 (Medi-Cal study)
Data on file, Janssen Medical Affairs, L.L.C., 2002 (Lash Group Study, CPT code 90782)
First DataBank price probe [online]. Available from URL: http://www.firstdatabank.com/reference-products/price-probe/ [Accessed 2003 Nov 1]
IMS Health National Disease and Therapeutic Index database [online]. Available from URL: http://www.imshealth.com [Accessed 2003 Sep 1]
Guest IF, Hart WM, Cookson RF, et al. Pharmacoeconomic evaluation of long-term treatment with risperidone for patients with chronic schizophrenia. Br J Med Econ 1996; 10: 59–67
Acknowledgements
The authors gratefully acknowledge the sponsorship funding provided by Janssen Medical Affairs, L.L.C.
Julie C. Locklear contributed to the construction and review of this manuscript.
Marcia Rupnow is an employee of Janssen Medical Affairs, L.L.C. At the time of writing this study, Natalie Edwards, Chris Pashos and Marc Botteman were employees of Abt Associates Inc., a company that provides research and consulting services under contract to pharmaceutical companies. As Project Director, Natalie Edwards conceived and led the project. Chris Pashos, as Executive Director and Vice President at Abt Associates, reviewed all material and served as an expert resource. Marc Botteman served as a Project Advisor and contributed to the initial study design and reviewed all material. Marcia Rupnow was the primary project contact at Janssen Medical Affairs, L.L.C. and provided input to the model and manuscript. Dr Ronald Diamond served as the clinical expert and focused mainly on reviewing the model and its input parameters for clinical accuracy and validity. Dr Diamond also provided valuable input in the writing of the study and ensuring its relevance from a clinical perspective.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, N.C., Rupnow, M.F.T., Pashos, C.L. et al. Cost-effectiveness model of long-acting risperidone in schizophrenia in the US. Pharmacoeconomics 23, 299–314 (2005). https://doi.org/10.2165/00019053-200523030-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200523030-00009