Abstract
Objective: To investigate the psychometric performance and clinical validity of the 36-Item Short Form (SF-36) health survey when completed by asymptomatic HIV-positive Italian patients and to compare their health profile with a representative sample of 2031 Italian citizens (the Italian norm).
Patients and Methods: This was an observational, multicentre, cross-sectional survey. Microbiologists throughout Italy recruited asymptomatic HIV-positive individuals who were aged at least 18 years and aware of their infection. Investigators collected demographic, social, clinical and treatment data. Patients, classified into 2 clinical categories (A1 and A2) according to explicit pre-defined criteria, completed the SF-36 health survey in the context of a medical visit.
Results: Between April and July 1996, 46 microbiologists recruited 214 patients (201 evaluable). No inconsistent responses were observed in 96% of the sample. The usually recommended psychometric standards were satisfied, and the internal consistency reliability indices were always greater than 0.70. Weak to moderate associations were found between SF-36 health survey scores and physicians’ estimates of patients’ physical performance, while no significant associations were found with CD4+ counts.
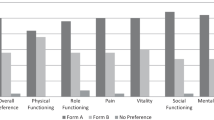
On average, HIV-positive patients reported lower scores than the Italian norm, and patients in category A2 showed lower scores than patients in A1. These differences were more relevant in scales describing role limitations, general health perception, and psychological well-being.
Conclusion: Our study showed that the SF-36 health survey maintained its psychometric properties in a sample of Italian asymptomatic HIV-positive patients and produced data that showed its validity and robustness in such a setting.








Similar content being viewed by others
References
Testa MA, Simonson DC. Assessment of quality of life outcomes. N Engl J Med 1996; 334: 835–40
Guyatt GH, Feeny DH, Patrick DL. Measuring health related quality of life. Ann Intern Med 1993; 118: 622–9
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995; 273: 59–65
Leplège A, Hunt S. The problem of quality of life in medicine. JAMA 1997; 278: 47–50
Apolone G. Defining and measuring quality of life in medicine [letter]. JAMA 1998; 279: 431
Spilker B, editor. Quality of life and pharmacoeconomics in clinical trial. 2nd ed. Philadelphia (PA): Lippincott-Raven Publishers, 1996
Stewart AL, Ware JE. Measuring functioning and well-being: the medical outcomes study approach. Durham (NC): Duke University Press, 1992
Tarlov AR, Ware JE, Greenfield S, et al. The medical outcomes study: an application of methods for monitoring the results of medical care. JAMA 1989; 262: 925–30
Donald LP, Deyo RA. Generic and disease specific measures in assessing health status and quality of life. Med Care 1989; 27: S217–31
Ware Jr JE, Sherbourne JD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care 1992; 30: 473–83
McHorney CA, Ware Jr JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–63
McHorney CA, Ware Jr JE, Lu FFR, et al. The MOS 36-Item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions and reliability across diverse patients groups. Med Care 1994; 32: 40–66
Ware Jr JE. SF-36 Health Survey: manual and interpretation guide. Boston (MA): The Health Institute, New England Medical Center, 1993
Berzon RA, Leplège AP, Lohr KN, et al. Summary and recommendations for future research. Qual Life 1997; 6: 601–5
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates, 1998
Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 1998; 51: 1025–36
Apolone G, Mosconi P, Ware Jr JE. Questionario sullo stato di salute SF-36: manuale d’uso e guida all’interpretazione dei risultati. Milan: Guerini e Associati Editore, 1997
1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep 1992 Dec 18; 41 (RR-17): 1–19
Karnofsky DA, Ableman WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948; 1: 634–56
Likert R. A technique for the measurement of attitudes. Arch Psychol 1932; 140: 5–55
Ware Jr JE, Kosnski M, Keller SD. SF-36 physical and mental health summary scales: a user’s manual. Boston (MA): The Health Institute, 1994
Ware Jr JE, Gandek B, Kosinski M, et al. The equivalence of the SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA project. J Clin Epidemiol 1998; 51: 1167–70
Wagner A, Gandek B, Aaronson NK, et al. Cross-cultural comparison of the content of SF-36 translations across fourteen countries. J Clin Epidemiol 1998; 51: 925–32
Keller SD, Ware J, Gandek B, et al. Testing the equivalence of widely-used translation of response choice labels: results from the IQOLA Project. J Clin Epidemiol 1998; 51: 933–44
Ware JE, Harris WJ, Gandek B, et al. MAP-R for Windows: multitrait/multi-item analysis program. Revised user’s guide. Boston (MA): Health Assessment, 1997
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951; 16: 297–334
Bland JM, Altman DG. Cronbach’s Alpha. BMJ 1997; 314: 572
Kazis LE, Anderson JJ, Meenan RF. Effect size for interpreting changes in health status. Med Care 1989; 27: 178–81
Kleinbaum DG, Lawrence LK, Keith EM, editors. Applied regression analysis and other multivariable methods. 2nd ed. Boston (MA): PWS-Kent Publishing Company, 1998
Goldfield N. The hubris of health status measurement: a clarification of its role in the assessment of medical care. Int J Qual Health Care 1996; 8: 115–23
Apolone G, Mosconi P. Health status assessment and managed care competition: are we on target? Int J Qual Health Care 1996; 8: 105–6
Acknowledgements
This study was fully supported by funds from Glaxo Wellcome Italy. Drs F. Arpinelli, G. Visonà and G. De Carli are employees of Glaxo Wellcome and Dr G. Apolone has served as a paid consultant to Glaxo Wellcome for the present study.
This research was possible thanks to the following researchers: Prof. F. Gritti, Bologna; Prof. B. De Rienzo, Modena; Dr L. Bonazzi, Reggio Emilia; Dr F. Alberici, Piacenza; Dr S. Ranieri, Ravenna; Prof. G. Scalise, Ancona; Prof.ssa M. Montroni, Ancona; Prof.ssa A. Orani, Lecco; Prof. G. Fiori, Varese; Prof. G. Filice, Pavia; Prof. L. Minoli, Pavia; Dr G. Carnevale, Cremona; Dr A. Cantaluppi, Lodi; Prof.ssa L. Cremoni, Monza; Prof.ssa L. Caggese, Milano; Prof. F. Suter, Busto Arsizio; Prof.ssa A. Cargnel, Milano; Prof. G. Angarano, Bari; Dr B. Grisorio, Foggia, Dr P. Grima, Galatina; Prof. P.E. Manconi, Cagliari; Prof. A. Aceti, Sassari; Dr C. D’Amato, Roma; Dr F. Soscia, Latina; Dr P. Franci, Roma; Prof. S. D’Elia, Roma; Prof. P. Cadrobbi, Padova; Prof. E. Raise, Venezia; Prof. F. De Lalla, Vicenza; Prof. U. Tirelli, Aviano; Prof. A. Chirianni, Napoli; Prof. M. Piazza, Napoli; Prof. F. Piccinino, Napoli; Prof. A. Nunnari, Catania; Dr B. Celesia, Catania; Prof. V. Abbadessa, Palermo; Dr.ssa S. Mancuso, Palermo; Dr G. Cassola, Genova; Dr G. Orofino, Torino; Dr.ssa S. Belloro, Torino; Dr A. Sinicco, Torino; Prof. F. Rizzo, Genova; Dr.ssa M.L. Soranzo, Torino; Prof. M. Della Santa, Pisa; Dr F. Mazzotta, Bagno a Ripoli; Prof. S. Pauluzzi, Perugia; Dr F. Leoncini, Firenze; Prof. G.P. Carosi, Brescia; Dr G. Cadeo, Brescia; Dr A. Scalzini, Mantova.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arpinelli, F., Visoná, G., Bruno, R. et al. Health-Related Quality of Life in Asymptomatic Patients with HIV. Pharmacoeconomics 18, 63–72 (2000). https://doi.org/10.2165/00019053-200018010-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200018010-00007