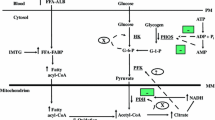
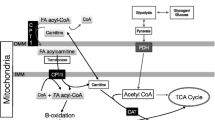
Abstract
Fat is an extremely important substrate for muscle contraction, both at rest and during exercise. Triglycerides (TGs), stored in adipose tissue and within muscle fibres, are considered to be the main source of the free fatty acids (FFAs) oxidised during exercise. It is still unclear, however, how the use of these substrates is regulated during exercise. The regulation seems to be multifactoral and includes: (i) dietary and nutritional status; (ii) hormonal milieu; (iii) exercise mode, intensity and duration; and (iv) training status.
On the other hand, the mechanism for FFA transport from its storage as triglycerides in adipose tissue and muscle to its place of utilisation in heart, skeletal muscle, kidney and liver is more clearly understood. It has been determined that the plasma FFA turnover rate is sufficiently rapid to account for most of the fat metabolised during low intensity exercise (25 to 40% V̇O2max). However, an exercise intensity of 65% V̇O2max results in a slight decrease in the amount of plasma FFA uptake by muscle tissue. Other studies have found that during prolonged exercise, muscle TGs become the predominant source of energy obtained from fat. Furthermore, it is widely documented that endurance activities increase the energy utilisation from fat while sparing carbohydrate sources. For example, during exercise on a cycle ergometer, nonplasma FFAs and plasma FFAs contribute 40%, and carbohydrates 60%, of the total calculated amount of energy expenditure before exercise and vice versa after exercise (60% nonplasma and plasma FFAs and 40% carbohydrates).
Although it was many years before it was fully demonstrated, fat is now known to be transported in the blood as FFA bound to the protein carrier albumin. The mobilisation of FFA is primarily a function of sympathetic nervous activity directed towards the adipocytes, or the ‘fat pad’. This nervous activity can be direct or may be an effect of circulating catecholamines such as adrenaline (epinephrine). This article summarises the role of fat metabolism during exercise.
Similar content being viewed by others
References
Chaveau A. Source et nature du potentiel directment utilise dans la travail musculaire d’apres les exchanges respiratoires, chez l’homme en etat d’abstinence. C R Acad Sci III 1896; 122: 1163–221
Zuntz A. Die Quellen der Muskelkraft. Appenheimer’s Handbuch Biochemie 1911; 4: 826–55
Fredrickson DS, Gordon Jr RS. The metabolism of albuminbound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest 1958; 37: 1504–15
Havel RJ, Naimark A, Borchgrevink CF. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1- C14. J Clin Invest 1963; 42: 1054–63
Huntford R. Scott and Amundsen. London: Hodder and Stoughton, 1979
Havel RJ, Carlson LA, Eklund L-G, et al. Turnover rate and oxidation of different fatty acids in man during exercise. J Appl Physiol 1964; 19: 613–9
Gollnick PD. Energy metabolism and prolonged exercise. In. Lamb DR, Murray R, eds. Perspectives in exercise science and sports medicine. Vol. 1. Indiana (IN): Benchmark Press, 1988: 1–36
Winder WW, Hickson RC, Hagberg JM, et al. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol 1979; 46: 766–71
Crampes F, Beauville M, Riviere D, et al. Effect of physical training in humans on the response of isolated fat cells to epinephrine. J Appl Physiol 1986; 61: 25–9
Seals DR, Victor RG, Mark AL. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol 1988; 65: 940–4
Cleroux J, Van Nguyen P, Taylor AW, et al. Effects of b2- vs b1- + b2-blockade on exercise endurance and muscle metabolism in humans. J Appl Physiol 1989; 66: 548–54
Henriksson J. Training-induced adaptations of skeletal muscle and metabolism during submaximal exercise. J Physiol (Lond) 1977; 270: 661–5
Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 1993; 265: E380–91
Carlson LA, Ekelund L-G, Froberg SO. Concentration of triglycerides, phospholipids and glycogen in skeletal muscle and of free fatty acids and b-hydroxybutyric acid in blood in man in response to exercise. Eur J Clin Invest 1971; 1: 248–54
Froberg SO, Mossfeldt F. Effect of prolonged strenuous exercise on the concentration of triglycerides, phospholipids and glycogen in muscle of man. Acta Physiol Scand 1971; 82: 167–71
Martin WH, Dalsky GP, Hurley BF, et al. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol 1993; 265: E708–14
Williams RS, Caron MG, Daniel K. Skeletal muscle b- adrenergic receptors: variations due to fiber type and training. Am J Physiol 1984; 246: E160–7
Terjung R, Mackie BG, Dudley GA, et al. Influence of exercise on chylomicron triacylglycerol metabolism: plasma turnover and muscle uptake. Med Sci Sports Exerc 1983; 15: 340–7
Wolfe RR, Klein S, Carraro F, et al. Role of triglyceride-fatty acid cycle in controlling fat metabolism during and after exercise. Am J Physiol 1990; 258: E382–9
Galbo H. Hormonal and metabolic adaptations to exercise. New York: Stratton, 1983: 5–7, 30-5, 64-9
Zierler KL. Fatty acids as substrates for heart and skeletal muscle. Circ Res 1976; 38: 459–63
Wahrenberg H, Lonnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest 1989; 84: 458–67
Kanaley JA, Mottram CD, Scanlon P, et al. Fatty acid kinetic responses to running above and below the lactate threshold. J Appl Physiol 1995; 79: 439–47
Lewis SF, Taylor WF, Graham RM, et al. Cardiovascular responses to exercise as functions of absolute and relative work load. J Appl Physiol 1983; 54: 1314–23
Issekutz B, Shaw BAS, Issekutz TB. Effect of lactate on FFA and glycerol turnover in resting and exercising dogs. J Appl Physiol 1975; 39: 349–53
Boyd AE, Gismber SR, Mager M, et al. Lactate inhibition of lipolysis in exercising man. Metabolism 1974; 23: 531–42
Galster AD, Clutter WE, Cryer PE, et al. Epinephrine plasma thresholds or lipolytic effects in man. J Clin Invest 1981; 67: 1729–38
Hartley LH, Manson JW, Hogan RP, et al. Multiple hormone responses to prolonged exercise in relation to physical training. J Appl Physiol 1972; 33: 607–10
Hurley BF, Nemeth PM, Martin III WH, et al. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol 1986; 60: 562–7
Miles JM, Jensen MD. Editorial: does glucagon regulate adipose tissue lipolysis?. J Clin Endocrinol Metab 1993; 77: 5A–6A
George JC, Jyoti D. The lipid content and its reduction in the muscle and liver during long and sustained muscular activity. J Anim Morphol Physiol 1955; 2: 31–7
Reitman J, Baldwin KM, Holloszy JO. Intramuscular triglyceride utilization by red, white, and intermediate skeletal muscle and heart during exhausting exercise. Proc Soc Exp Biol Med 1973; 142: 628–31
Turcotte LP, Richer EA, Kiens B. Increased plasma FFA uptake and oxidation during exercise in trained vs untrained humans. Am J Physiol 1992; 262: E791–9
Havel RJ, Pernow B, Jones NL. Uptake and release of fatty acids and other substrates in the legs of exercising men. J Appl Physiol 1967; 23: 90–9
Kiens B, Essen-Gustavsson B, Christensen NJ, et al. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol (Lond) 1993; 469: 459–78
Coggan AR, Kohrt WM, Spina RJ, et al. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol 1990; 68: 990–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranallo, R.F., Rhodes, E.C. Lipid Metabolism During Exercise. Sports Med 26, 29–42 (1998). https://doi.org/10.2165/00007256-199826010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-199826010-00003