Summary
Abstract
Bivalirudin (Angiox™, Angiomax®) is a synthetic 20-amino acid peptide analogue of hirudin. It is a direct thrombin inhibitor that binds specifically and reversibly to both fibrin-bound and unbound thrombin. Intravenous bivalirudin is approved in Europe for use as an anticoagulant in patients undergoing percutaneous coronary intervention (PCI). In the US, bivalirudin is approved in patients with unstable angina pectoris undergoing percutaneous transluminal coronary angioplasty (PTCA) and has recently been approved for use with provisional glycoprotein (GP) IIb/IIIa inhibition in patients undergoing PCI.
Bivalirudin plus provisional GP IIb/IIIa inhibition is effective in patients undergoing PCI. The large, well controlled REPLACE-2 (Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events) study showed that bivalirudin plus provisional GP IIb/IIIa inhibition was noninferior to heparin plus planned GP IIb/IIIa inhibition and that bivalirudin was associated with a reduced risk of bleeding complications. In patients with heparin-induced thrombocytopenia (HIT), bivalirudin was effective against ischaemic events and there was a low incidence of bleeding complications. Bivalirudin should be considered as an alternative to heparin plus planned GP IIb/IIIa inhibition in any patient undergoing urgent or elective PCI, especially in any patient with a high risk of bleeding complications.
Pharmacological Properties
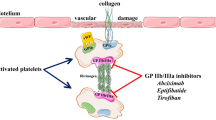
Bivalirudin directly inhibits thrombin by binding to the active site and exosite 1 of thrombin. This binding is specific, noncompetitive and initially irreversible. The inhibition is reversed as bivalirudin is slowly cleaved by thrombin, freeing the active site and allowing thrombin to regain its activity.
Intravenous administration of bivalirudin results in a dose-dependent increase in anticoagulation parameters. Bivalirudin inhibits thrombin generation and activity, as well as thrombin-mediated platelet activation.
Bivalirudin does not bind to plasma proteins other than thrombin and has a small volume of distribution. It is cleared from plasma by a combination of proteolytic cleavage and renal mechanisms; bivalirudin plasma clearance is affected by impaired renal function. In patients with normal renal function, bivalirudin is rapidly cleared from plasma and has a terminal half-life of 25 minutes.
Therapeutic Efficacy
The REPLACE-2 trial in patients undergoing PCI found that bivalirudin plus provisional GP IIb/IIIa inhibition was noninferior to heparin plus planned GP IIb/IIIa inhibition for the composite primary endpoint of death, myocardial infarction (MI), repeat revascularisation or major bleeding at 30 days, or for the secondary endpoint of death, MI or repeat revascularisation. There was no between-group difference in the incidence of all-cause mortality after 1 year.
Similar outcomes were observed in REPLACE-2 substudies of special patient groups, including patients with impaired renal function, diabetes mellitus or acute coronary syndromes, and in other studies in patients undergoing brachytherapy or patients with HIT.
Tolerability
Bivalirudin has been associated with a lower incidence of bleeding complications than heparin. In the REPLACE-2 trial, there was a significant 41% relative reduction in the incidence of major bleeding in the bivalirudin plus provisional GP IIb/IIIa inhibitor group compared with the heparin plus planned GP IIb/IIIa inhibitor group (2.4% vs 4.1%; p < 0.001).
The incidence of major bleeding was lower in bivalirudin recipients than heparin recipients across most analysed subgroups of patients including those with impaired renal function or anaemia and female patients. The incidence of bleeding events was also low in a noncomparative trial of bivalirudin in patients with HIT.
Bivalirudin was generally well tolerated. In REPLACE-2, the most frequent adverse events in bivalirudin recipients were back pain, angina pectoris, pain, hypotension and nausea.
Pharmacoeconomic Considerations
Total 30-day costs were lower for bivalirudin plus provisional GP IIb/IIIa inhibitor recipients than for heparin plus planned GP IIb/IIIa inhibitor recipients ($US10 868 vs $US11 242; year of costing 2002) in REPLACE-2. Approximately 80% of the cost reduction was due to a difference in anticoagulation cost (between bivalirudin and GP IIb/IIIa inhibitors) and 20% was due to differences in the incidences of ischaemic and haemorrhagic complications.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Gladwell TD. Bivalirudin: a direct thrombin inhibitor. Clin Ther 2002 Jan; 24(1): 38–58
Simonton C. Direct thrombin inhibitors. J Invasive Cardiol 2004 Jul; 16 (7 Suppl.): 17S–23S
Nikolsky E, Dangas GD. Percutaneous interventions in patients with immune-mediated heparin-induced thrombocytopenia. Semin Thromb Hemost 2004 Jun; 30(3): 305–14
Wiggins BS, Spinler S, Wittkowsky AK, et al. Bivalirudin: a direct thrombin inhibitor for percutaneous transluminal coronary angioplasty. Pharmacotherapy 2002 Aug; 22(8): 1007–18
Carswell CI, Plosker GL. Bivalirudin: a review of its potential place in the management of acute coronary syndromes. Drugs 2002; 62(5): 841–70
Nappi J. The biology of thrombin in acute coronary syndromes. Pharmacotherapy 2002; 22 (6 Pt 2): 90S–6S
Bates ER. Bivalirudin: an anticoagulant option for percutaneous coronary intervention. Expert Rev Cardiovasc Ther 2004 Mar; 2(2): 153–62
Warkentin TE. Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol 2004 Mar; 17(1): 105–25
Parry MAA, Maraganore JM, Stone SR. Kinetic mechanism for the interaction of hirulog with thrombin. Biochemisty 1994 Dec 13; 33(49): 14807–14
Fox I, Dawson A, Loynds P, et al. Anticoagulant activity of hirulog, a direct thrombin inhibitor, in humans. Thromb Haemost 1993 Feb 1; 69(2): 157–63
Lidón R-M, Théroux P, Juneau M, et al. Initial experience with a direct antithrombin, hirulog, in unstable angina: anticoagulant, antithrombotic, and clinical effects. Circulation 1993 Oct; 88 (4 Pt 1): 1495–501
Fuchs J, Cannon CP. Hirulog in the treatment of unstable angina: results of the Thrombin Inhibition in Myocardial Ischemia (TIMI) 7 trial. Circulation 1995 Aug 15; 92(4): 727–33
Sharma GV, Lapsley D, Vita JA, et al. Usefulness and tolerability of hirulog, a direct thrombin-inhibitor, in unstable angina pectoris. Am J Cardiol 1993 Dec 15; 72(18): 1357–60
Topol EJ, Bonan R, Jewitt D, et al. Use of a direct antithrombin, hirulog, in place of heparin during coronary angioplasty. Circulation 1993 May; 87(5): 1622–9
Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003 Feb 19; 289(7): 853–63
Kleiman NS, Klem J, Fernandes LS, et al. Pharmacodynamic profile of the direct thrombin antagonist bivalirudin given in combination with the glycoprotein IIb/IIIa antagonsit eptifibatide. Am Heart J 2002 Apr; 143(4): 585–93
Aggarwal A, Sobel BE, Schneider DJ. Decreased platelet reactivity in blood anticoagulated with bivalirudin or enoxaparin compared with unfractionated heparin: implications for coronary intervention. J Thromb Thrombolysis 2002 Jun; 13(3): 161–5
Robson R, White H, Aylward P, et al. Bivalirudin pharmacokinetics and pharmacodynamics: effect of renal function, dose, and gender. Clin Pharmacol Ther 2002 Jun; 71(6): 433–9
Irvin W, Sica D, Gehr T, et al. Pharmacodynamics (PD) and kinetics (PK) of bivalirudin (BIV) in renal failure (RF) and hemodialysis (HD) [abstract no PIII-104]. Clin Pharmacol Ther 1999 Feb; 65(2): 202
Robson R. The use of bivalirudin in patients with renal impairment. J Invasive Cardiol 2000 Dec; 12 Suppl. F: 33F–6F
Koster A, Spiess B, Chew DP, et al. Effectiveness of bivalirudin as a replacement for heparin during cardiopulmonary bypass in patients undergoing coronary artery bypass grafting. Am J Cardiol 2004 Feb 1; 93(3): 356–9
Angiox: European public assessment report and product information [online]. Available from URL: http://www.emea.eu.int/humandocs/Humans/EPAR/angiox/angioxM.htm [Accessed 2005 Mar 29]
Lincoff AM, Bittl JA, Kleiman NS, et al. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to reduced Clinical Events [REPLACE]-1 Trial). Am J Cardiol 2004 May 1; 93(9): 1092–6
The Medicines Company. Angiomax (bivalirudin): prescribing information. Parsippany (NJ): The Medicines Company, 2005 Jun 14
Bittl JA, Strony J, Brinker JA, et al. Treatment with bivalirudin (hirulog) as compared with heparin during coronary angioplasty for unstable or postinfarction angina. Hirulog Angioplasty Study Investigators. N Engl J Med 1995 Sep 21; 333(12): 764–9
Bittl JA, Chaitman BR, Feit F, et al. Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J 2001 Dec; 142(6): 952–9
Lincoff AM, Kleiman NS, Kottke-Marchant K, et al. Bivalirudin with planned or provisional abciximab versus lowdose heparin and abciximab during percutaneous coronary revascularization: results of the Comparison of Abciximab Complications with Hirulog for Ischemic Events Trial (CACHET). Am Heart J 2002 May; 143(5): 847–53
Lincoff AM, Kleiman NS, Kereiakes DJ, et al. Long-term efficacy of bivalirudin and provisional glycoprotein IIb/IIIa blockade vs heparin and planned glycoprotein Ilb/IIIa blockade during percutaneous coronary revascularization: REPLACE-2 randomized trial. JAMA 2004 Aug 11; 292(6): 696–703
Saw J, Lincoff AM, DeSmet W, et al. Lack of clopidogrel pretreatment effect on the relative efficacy of bivalirudin with provisional glycoprotein IIb/IIIa blockade compared to heparin with routine glycoprotein IIb/IIIa blockade: a REPLACE-2 substudy. J Am Coll Cardiol 2004 Sep 15; 44(6): 1194–9
Chew DP, Lincoff AM, Gurm H, et al. Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the REPLACE-2 trial). Am J Cardiol 2005 Mar 1; 95(5): 581–5
Chew DP, Bhatt DL, Kimball W, et al. Bivalirudin provides increasing benefit with decreasing renal function: a meta-analysis of randomized trials. Am J Cardiol 2003 Oct 15; 92(8): 919–23
Kesanakurthy S, Mehran R, Tchelebi A, et al. Superior clinical outcomes with bivalirudin during intracoronary brachytherapy [abstract no. TCT-406]. Am J Cardiol2003; 92 (6 Suppl.): 172L
Awar M, Kabous N, Patel T, et al. Safety of bivalirudin during brachytherapy with beta radiation [abstract no. A30]. 26th Annual Scientific Sessions of the Society for Cardiac Angiography and Interventions (SCAI); May 7–10 2003; Boston (MA)
Wolfram R, Kuchulakanti PK, Rha SW, et al. Safety of bivalirudin as a single antithrombotic agent in patients undergoing percutaneous coronary intervention and vascular brachytherapy with gamma and beta emitters [abstract no. 1063-62]. J Am Coll Cardiol 2004 Mar 3; 43 (5 Suppl. A): 52A
Gurm HS, Sarembock IJ, Kereiakes DJ, et al. Use of bivalirudin during percutaneous coronary intervention in patients with diabetes mellitus: an analysis from the randomized evaluation in percutaneous coronary intervention linking angiomax to reduced clinical events (REPLACE)-2 trial. J Am Coll Cardiol 2005 Jun 21; 45(12): 1932–8
Rajagopal V, Lincoff AM, Cohen DJ, et al. Outcomes of patients with acute coronary syndromes who are treated with bivalirudin during PCI: an analysis from the REPLACE-2 trial [abstract no. 1624]. Circulation 2004 Oct 26; 110 (17 Suppl.): III–340
Mahaffey KW, Lewis BE, Wildermann NM, et al. The anticoagulant therapy with bivalirudin to assist in the performance of percutaneous coronary intervention in patients with heparin-induced thrombocytopenia (ATBAT) study: main results. J Invasive Cardiol 2003 Nov; 15(11): 611–6
Rha S-W, Kuchulakanti PK, Pakala R, et al. Bivalirudin versus heparin as an antithrombotic agent in patients treated with a sirolimus-eluting stent. Am J Cardiol 2004 Oct 15; 94(8): 1047–50
Dangas G, Lasic Z, Mehran R, et al. Effectiveness of the concomitant use of bivalirudin and drug-eluting stents (from the prospective, multicenter BivAlirudin and Drug-Eluting STents [ADEST] study). Am J Cardiol. In Press
Gurm HS, Rajagopal V, Fathi R, et al. Effectiveness and safety of bivalirudin during percutaneous coronary intervention in a single medical center. Am J Cardiol 2005 Mar 15; 95: 716–21
Barman N, Gurm H, Bhatt D, et al. Clinical outcomes associated with the use of bivalirudin for patients with acute coronary syndrome undergoing early percutaneous coronary intervention [abstract no. TCT-408]. Am J Cardiol 2004 Sep 30; 94 (6 Suppl.): 191E
Mehran R, Aymong E, Moses J, et al. Early clinical benefit with bivalirudin compared to unfractionated heparin during percutaneous coronary intervention [abstract no. TCT-309]. Am J Cardiol 2003; 92 (6 Suppl.): 134L
Hodgson JM, Bauchens KJ, Clark C, et al. Bivalirudin in routine clinical practice: concurrent evaluation shows reduced complications compared with heparin-based thrombin inhibition [abstract no. TCT-301]. Am J Cardiol 2003; 92 (6 Suppl.): 130L
Jennings HR, Poe KL, Carrico JM, et al. Percutaneous coronary intervention outcomes and the impact of pharmacologic selection: glycoprotein IIbIIIa inhibitors versus bivalirudin [abstract no. 1039-29]. J Am Coll Cardiol 2005 Feb 1; 45 Suppl. A: 37A
Ebrahimi R, Lincoff AM, Shah AP, et al. Bivalirudin versus heparin in percutaneous coronary interventions: a meta-analysis [abstract no. 1039-25]. J Am Coll Cardiol 2005 Feb 1; 45 Suppl. A: 36A
Rao AK, Pratt C, Berke A, et al. Thrombolysis in myocardial infarction (TIMI) trial-phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol 1988 Jan; 11(1): 1–11
Bhatt DL, Cho L, Lincoff AM, et al. Reduction in percutaneous coronary intervention: related bleeding with bivalirudin is particularly striking in women [abstract no. 1053-4]. J Am Coll Cardiol 2002 Mar 6; 39 (5 Suppl. A): 17A
Tsuchiya Y, Lansky A, Costa RA, et al. Gender and outcomes after percutaneous coronary intervention in patients treated with bivalirudin and provisional glycoprotein IIb/IIIa blockade versus heparin and planned glycoprotein IIb/IIIa blockade: results from the REPLACE-2 trial [abstract no. TCT-231]. Am J Cardiol 2004 Sep 30; 94 (6 Suppl.): 106E–7E
Voeltz MD, Attubato MJ, Feit F, et al. Anemia is associated with increased major bleeding complications and early mortality in patients undergoing percutaneous coronary intervention: implications for choices in antithrombotic therapy [abstract no. 1037-13]. J Am Coll Cardiol 2005 Feb 1; 45 (3 Suppl. A): 31A
Bittl JA. Comparative safety profiles of hirulog and heparin in patients undergoing coronary angioplasty. Am Heart J 1995 Sep; 130 (3 Pt 2): 658–65
Milkovich G, Gibson G. Economic impact of bleeding complications and the role of antithrombotic therapies in percutaneous coronary intervention. Am J Health Syst Pharm 2003 Jul 15; 60 (14 Suppl. 3): S15–21
Compton A. A practical cost analysis of bivalirudin. Pharmacotherapy 2002 Jun; 22 (6 Pt 2): 119S–27S
Cohen DJ, Lincoff AM, Lavelle TA, et al. Economic evaluation of bivalirudin with provisional glycoprotein IIb/IIIa inhibition versus heparin with routine glycoprotein IIb/IIIa inhibition for percutaneous coronary intervention: results from the REPLACE-2 trial. J Am Coll Cardiol 2004 Nov 2; 44(9): 1792–800
Schussler JM, Cameron CS, Anwar A, et al. Effect of bivalirudin on length of stay in the recovery area after percutaneous coronary intervention compared with heparin alone, heparin + abciximab, or heparin + eptifibatide. Am J Cardiol 2004 Dec 1; 94(11): 1417–9
Ormiston JA, Shaw BL, Panther MJ, et al. Percutaneous coronary intervention with bivalirudin anticoagulation, immediate sheath removal, and early ambulation: a feasibility study with implications for day-stay procedures. Cathet Cardiovasc Interv 2002 Mar; 55(3): 289–93
Minutello RM, Wong SC, Chou ET, et al. Angiomax facilitates early sheath removal after coronary angioplasty: the AFRICA study [abstract no. TCT-340]. Am J Cardiol 2003; 92 (6 Suppl.): 146L
Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003 Oct 15; 92(8): 930–5
Silber S, Albertsson P, Avilés FF, et al. Guidelines for percutaneous coronary interventions: the task force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J 2005 Apr; 26(8): 804–47
Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction-2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). Circulation 2002 Oct 1; 106(14): 1893–900
Kokolis S, Cavusoglu E, Clark LT, et al. Anticoagulation strategies for patients undergoing percutaneous coronary intervention: unfractionated heparin, low-molecular-weight heparins, and direct thrombin inhibitors. Prog Cardiovasc Dis 2004 May–June; 46(6): 506–23
Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy and safety. Chest 2001 Jan; 119 (1 Suppl.): 64S–94S
Antman EM. Should bivalirudin replace heparin during percutaneous coronary interventions? JAMA 2003 Feb 19; 289(7): 903–5
Benefits and controversies: the REPLACE-2 trial [interview with REPLACE-2 investigator Michael Attubato, MD]. Cath Lab Digest 2003; 11 (4)
Maroo A, Lincoff AM. Bivalirudin in PCI: an overview of the REPLACE-2 trial. Semin Thromb Hemost 2004 Jun; 30(3): 329–36
Ioannidis JPA, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol 2003 Oct 15; 42(8): 1406–11
Lopez LM. Clinical challenges of bleeding in percutaneous coronary intervention. Am J Health Syst Pharm 2003 Jul 15; 60 (14 Suppl. 3): S8–S14
Lincoff AM, Bittl JA, Harrington RA, et al. Evolving role of bivalirudin in percutaneous coronary interventions; impact of the REPLACE-2 study. Expert Opin Invest Drugs 2003 Jun; 12(6): 1027–33
Stone GW, Bertrand M, Colombo A, et al. Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale. Am Heart J 2004 Nov; 148(5): 764–75
Bivalirudin in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention (BIAMI trial) [online]. Available from URL: http://www.clinicaltrials.gov/ct/show/NCT00093184?order=3 [Accessed 2005 May 20]
HORIZONS trial [online]. Available from URL: http://www.lebauercvresearch.org/ [Accessed 2005 Jul 26]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: E.R. Bates, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA; J.A. Bittl, Ocala Heart Institute, Munroe Regional Medical Center, Florida, USA; J. Brinker, Division of Cardiology, Johns Hopkins Hospital, Baltimore, USA; H. Gurm, Division of Cardiology, University of Michigan Health System, Ann Arbor, Michigan, USA; N.S. Kleiman, Baylor College of Medicine, The Methodist Hospital, Houston, Texas, USA; A.M. Lincoff, Department of Cardiovascular Medicine, The Cleveland Clinic Foundation, Cleveland, Ohio, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on bivalirudin, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘bivalirudin’ or ‘hirulog’. Searches were last updated 8 August 2005.
Selection: Studies in patients undergoing percutaneous coronary intervention who received bivalirudin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Bivalirudin, percutaneous coronary intervention, percutaneous transluminal coronary angioplasty, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Moen, M.D., Keating, G.M. & Wellington, K. Bivalirudin. Drugs 65, 1869–1891 (2005). https://doi.org/10.2165/00003495-200565130-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565130-00010