Summary
Abstract
Irbesartan (Avapro®, Aprovel®) is a potent and selective angiotensin II subtype 1 receptor antagonist indicated for use in patients with hypertension, including those with type 2 diabetes mellitus and nephropathy.
Once-daily administration of irbesartan provided 24-hour control of blood pressure (BP). In patients with mild-to-moderate hypertension irbesartan was as effective as enalapril, atenolol and amlodipine, and more effective than valsartan in terms of absolute reduction in BP and response rates. Irbesartan produced a greater reduction in diastolic BP at trough than once-daily losartan, but had a smaller effect than olmesartan; the reduction in systolic BP achieved with irbesartan was similar or greater than that with losartan and similar to that seen with olmesartan. The combination of irbesartan with hydrochlorothiazide produced additive effects on BP reduction. Irbesartan also induced regression of left ventricular mass in patients with hypertension and left ventricular hypertrophy.
In two large studies (IRbesartan MicroAlbuminuria type 2 diabetes mellitus in hypertensive patients [IRMA 2] and the Irbesartan Diabetic Nephropathy Trial [IDNT]) irbesartan exerted a renoprotective effect in hypertensive patients with type 2 diabetes at both the early and later stages of diabetic nephropathy. The renoprotective effect was at least partly independent of the BP-lowering effect. In the IRMA 2 trial, the proportion of patients progressing to overt nephropathy was significantly lower for recipients of irbesartan 300mg once daily than placebo. In patients with overt nephropathy in the IDNT, irbesartan 300mg once daily provided significantly greater renoprotection than amlodipine 10mg once daily or placebo. The relative risk of doubling of serum creatinine was significantly lower with irbesartan than amlodipine or placebo.
Irbesartan is well tolerated in hypertensive patients, including those with type 2 diabetes and incipient or overt nephropathy. The overall incidence of adverse events with irbesartan was similar to that with placebo. Irbesartan was associated with a lower incidence of cough than enalapril and was not associated with ankle oedema or with any clinically significant drug interactions.
In conclusion, irbesartan is a well tolerated and effective antihypertensive agent. It also slows the progression of renal disease in hypertensive patients with type 2 diabetes at both the early and later stages of diabetic nephropathy. Thus, irbesartan is a valuable agent in the management of patients with these indications.
Pharmacodynamic Properties
Irbesartan selectively binds to the angiotensin II receptor subtype 1 (AT1), inhibiting the activity of angiotensin II. Studies in normotensive volunteers indicate that irbesartan exerts a long-lasting inhibitory effect on the pressor response to exogenous angiotensin II. Irbesartan 5–300mg induced elevations in plasma renin activity and angiotensin II levels in volunteers with or without renal failure. In healthy volunteers, the AT1 blockade induced by irbesartan was generally significantly greater and of longer duration than that induced by losartan and valsartan. While irbesartan and candesartan demonstrated a similar extent of AT1 antagonistic activity in vivo, ex vivo/in vitro studies suggested higher antagonistic activity for irbesartan. A greater reduction in aldosterone levels and a greater increase in plasma renin activity occurred with irbesartan than with candesartan.
Systolic and diastolic blood pressure (BP) did not change significantly after single doses of irbesartan 5–300mg in salt-replete normotensive volunteers, but decreased following single doses of irbesartan 10–100mg in salt-depleted volunteers. Heart rate was unaltered.
In healthy volunteers and hypertensive patients, irbesartan 50mg and 100mg, respectively, increased renal blood flow and decreased renal vascular resistance without affecting the glomerular filtration rate. Irbesartan 50mg increased sodium excretion in normotensive volunteers, without altering potassium or uric acid excretion.
Pharmacokinetic Properties
The mean bioavailability of irbesartan is approximately 60–80% after oral administration and is not affected by food. After administration of single or multiple doses of irbesartan 150–300mg, maximum plasma drug concentrations (Cmax) were achieved within 1.5–2 hours. Values for Cmax and area under the concentration-time curve (AUC) are dose-related within this range and values are similar after single or multiple doses. Irbesartan is ≥90% bound to plasma proteins and has a steady-state volume of distribution of 53–93L.
The primary metabolic fate of irbesartan is oxidation via cytochrome P450 (CYP) isoform 2C9. Metabolism by CYP3A4 is negligible. The pharmacological activity of metabolites is minimal. After oral administration to healthy volunteers <10% and 30% of the parent drug was recovered in urine and faeces, respectively. Total plasma clearance of irbesartan was approximately 9–11 L/h, of which approximately 0.18 L/h was renal clearance. The elimination half-life of multiple doses of irbesartan 150 or 300mg was 11 hours.
No differences were noted in the pharmacokinetic properties of irbesartan between healthy men and women, but the AUC and Cmax were higher in elderly than younger volunteers. The pharmacokinetic parameters of irbesartan are not significantly altered by mild-to-moderate or severe renal impairment, mild-to-moderate hepatic impairment or heart failure.
No significant and clinically relevant drug interactions have been identified between irbesartan and hydrochlorothiazide, nifedipine, simvastatin, tolbutamide, warfarin, magnesium and aluminium hydroxides or digoxin. Fluconazole increased the irbesartan Cmax and AUC by 19% and 63%.
Therapeutic Efficacy in Hypertension
Monotherapy with irbesartan 150–300mg once daily was effective at reducing BP in patients with hypertension in double-blind, placebo- or active-controlled studies, leading to placebo-subtracted reductions in trough seated BP of approximately 8–10/5-6mm Hg. Reductions in BP were maintained over a 24-hour period with once-daily dosing. Irbesartan reduced BP by 11–19/7–13mm Hg in active-controlled clinical trials compared with reductions of 8–18/5–14mm Hg with the comparator drugs. Response rates with irbesartan were 36–72% compared with 43–68% for comparators.
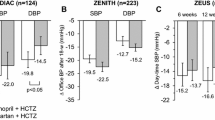
Irbesartan 150mg once daily produced significantly greater reductions in diastolic and systolic BP and higher response and normalisation rates than valsartan 80mg once daily in the only study involving both these agents to include a statistical comparison. Reductions in diastolic BP with irbesartan 150–300mg once daily were greater than with losartan 50–100mg once daily, although the effect on systolic BP was significantly greater for irbesartan in only 1 of 2 studies, and there were no significant differences in response or normalisation rates between the drugs. Irbesartan 150mg once daily reduced diastolic BP to a lesser extent than olmesartan 20mg once daily, but produced similar changes in systolic BP and mean 24-hour ambulatory BP to olmesartan.
Several trials have shown irbesartan to be as effective as enalapril 10–20mg once daily in patients with mild-to-moderate hypertension, including in patients aged ≥65 years. Single studies have shown irbesartan 75–150mg once daily to produce similar decreases in BP to atenolol 50–100mg once daily, and irbesartan 150mg once daily to be as effective as amlodipine 5mg once daily.
The combination of irbesartan 75–300mg once daily with hydrochlorothiazide 12.5–25mg once daily produced additive BP-lowering effects in patients with mild-to-moderate hypertension. Irbesartan 150mg combined with hydrochlorothiazide 12.5mg (both once daily) produced reductions in BP of 4–7/2–4mm Hg additional to those with the individual components. The combination reduced BP in patients whose BP was unresponsive to monotherapy with irbesartan or hydrochlorothiazide.
Irbesartan induced regression of left ventricular mass in patients with left ventricular hypertrophy in several trials. The reduction in left ventricular mass was significantly greater with irbesartan than with amlodipine or atenolol, despite reductions in BP being similar for irbesartan and the comparators.
Therapeutic Efficacy in Hypertensive Patients with Type 2 Diabetes Mellitus and Nephropathy
Data from two large randomised, double-blind, placebo-controlled studies [Irbesartan Microalbuminuria Type 2 Diabetes Mellitus in hypertensive patients (IRMA 2) and the Irbesartan Diabetic Nephropathy Trial (IDNT)] showed that irbesartan slowed the development of overt nephropathy and the progression of renal disease in hypertensive patients with type 2 diabetes, and suggested that the renoprotective effect of irbesartan is at least in part independent of its BP-lowering effect.
In the IRMA 2 trial in patients with microalbuminuria (20–200 μg/min) and normal kidney function, overt nephropathy developed in 5% of irbesartan 300mg once daily recipients (p < 0.001 vs placebo), 10% of irbesartan 150mg once daily recipients (not significant) and 15% of placebo recipients. The hazard ratio for diabetic nephropathy was 0.3 in recipients of irbesartan 300mg once daily but did not achieve statistical significance in recipients of irbesartan 150mg once daily. Normoalbuminuria was restored in significantly more patients receiving irbesartan 300mg, but not 150mg, once daily compared with placebo. The renoprotective effect of irbesartan 300mg once daily was sustained in patients for whom antihypertensive medication was withdrawn for 1 month after completing 2 years of treatment.
The average trough systolic but not diastolic BP was significantly lower for the combined irbesartan groups than placebo (by l–3mm Hg). Reductions in ambulatory BP, measured in a subset of patients, were similar between groups.
In patients with more advanced renal disease, including proteinuria and elevated serum creatinine levels (IDNT), the relative risk of reaching the primary endpoint (a composite measure of doubling of the serum creatinine level, end-stage renal disease [ESRD] or death) was significantly lower in recipients of irbesartan 300mg once daily than placebo (by 20%) and amlodipine 10mg once daily (by 23%). The relative risk of doubling of serum creatinine levels and the rate at which serum creatinine levels increased were significantly lower in patients receiving irbesartan than placebo or amlodipine recipients. The relative risk of progression to ESRD alone was 23% lower in irbesartan recipients than in placebo or amlodipine recipients (not significant). The mean arterial BP in irbesartan recipients was not significantly different to that in amlodipine recipients.
Simulations of the long-term cost consequences of treatment with irbesartan, based on the outcomes of the IDNT, predicted that compared with amlodipine over a 25-year (lifetime) horizon irbesartan would be associated with an increase in life expectancy of 0.34–0.71 years and per-patient cost savings of €21 163-27 044 in the Belgian or French settings (year of costing 2002), $US26 290 in the US (year of costing 2000) and $Can 19 976 in Canada (year of costing 2001).
Tolerabilitv
There was no clinically relevant difference between irbesartan ≤900mg/day and placebo in the overall incidence of adverse drug events (21% vs 20%) in a pooled analysis of nine placebo-controlled trials. The incidence of withdrawal because of adverse events was 3.3% in irbesartan recipients and 4.5% in placebo recipients. Musculoskeletal trauma was the only adverse event to occur significantly more requently in recipients of irbesartan than placebo (1.9% vs 0.5%, p < 0.05).
Analysis of tolerability data from five nonblind trials (mean duration 276 days) involving 1006 patients with hypertension indicated that irbesartan monotherapy (≤300mg once daily) was well tolerated. In this analysis, the incidence of adverse drug experiences was 20% in recipients of irbesartan monotherapy, with 6% of recipients of irbesartan monotherapy discontinuing because of adverse events.
The overall incidence of adverse events with irbesartan was similar to that of most comparator agents. However, irbesartan was associated with a significantly lower incidence of cough than enalapril and was not associated with ankle oedema. The combination of irbesartan and hydrochlorothiazide was also well tolerated in patients with hypertension, with the overall incidence of adverse events being similar to that for placebo.
In the IRMA 2 trial, serious adverse events occurred in fewer irbesartan than placebo recipients (15% vs 23%). In the IDNT, the rate of adverse events per 1000 treatment days was significantly lower in recipients of irbesartan than placebo or amlodipine. The number of patients experiencing at least one serious adverse event did not differ significantly between the treatment groups. Significantly more irbesartan patients (1.9%) withdrew because of hyperkalaemia than amlodipine (0.5%) or placebo (0.4%) recipients. Patients treated with irbesartan reported more orthostatic symptoms than those receiving placebo.
Dosaae and Administration
Irbesartan is approved for the treatment of hypertension in many countries worldwide. In a number of countries, including the US, Canada, Australia and in Europe, it has also been approved for the treatment of nephropathy in patients with type 2 diabetes and hypertension.
Irbesartan may be used alone or in combination with other antihypertensive agents. The usual starting dosage is 150mg administered once daily, and the maximum recommended dosage for both indications is 300mg once daily, which is also the recommended maintenance dosage for patients with diabetic nephropathy. Irbesartan may be administered with or without food. No dosage adjustments are necessary in patients with hepatic or renal impairment. Dosage adjustments are not usually necessary in elderly patients, but consideration may be given to starting treatment at 75mg once daily in those aged >75 years. Irbesartan should not be administered during the second and third trimesters of pregnancy.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Gillis JC, Markham A. Irbesartan: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in the management of hypertension. Drugs 1997 Dec; 54(6): 885–902
Markham A, Spencer CM, Jarvis B. Irbesartan: an update of its use in cardiovascular disorders. Drugs 2000 May; 59(5): 1187–206
Burnier M. Angiotensin II type 1 receptor blockers. Circulation 2001 Feb 13; 103(6): 904–12
Ellis ML, Patterson JH. A new class of antihypertensive therapy: angiotensin II receptor antagonists. Pharmacotherapy1996 Sep–Oct; 16(5): 849–60
White M, Racine N, Ducharme A, et al. Therapeutic potential of angiotensin II receptor antagonists. Expert Opin Invest Drugs 2001 Sep; 10(9): 1687–701
Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: Clinical relevance and therapeutic role [published erratum appears in Am J Health Syst Pharm 2001 Sep 1; 58 (17): 1168]. Am J Health Syst Pharm 2001 Apr; 58(8): 671–83
Belz GG, Butzer R, Kober S, et al. Time course and extent of angiotensin II antagonism after irbesartan, losartan, and valsartan in humans assessed by angiotensin II dose response and radioligand receptor assay. Clin Pharmacol Ther 1999 Oct; 66(4): 367–73
Mazzolai L, Maillard M, Rossat J, et al. Angiotensin II receptor blockade in normotensive subjects: a direct comparison of three AT1 receptor antagonists. Hypertension 1999 Mar; 33(3): 850–5
Ribstein J, Picard A, Armagnac C, et al. Inhibition of the acute effects of angiotensin II by the receptor antagonist irbesartan in normotensive men. J Cardiovasc Pharmacol 2001 Apr; 37(4): 449–60
Burnier M, Hagman M, Nussberger J, et al. Short-term and sustained renal effects of angiotensin II receptor blockade in healthy subjects. Hypertension 1995 Apr; 25 (4 Pt 1): 602–9
Schmitt F, Martinez F, Brillet G, et al. Acute renal effects of AT1-receptor blockade after exogenous angiotensin II infusion in healthy subjects. J Cardiovasc Pharmacol 1998 Feb; 31(2): 314–21
Marino MR, Langenbacher K, Ford NF, et al. Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol 1998 Mar; 38(3): 246–55
Pechère-Bertschi A, Nussberger J, Decosterd L, et al. Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens 1998 Mar; 16(3): 385–93
Sica DA, Marino MR, Hammett JL, et al. The pharmacokinetics of irbesartan in renal failure and maintenance hemodialysis. Clin Pharmacol Ther 1997 Dec; 62(6): 610–8
Butzer R, Belz GG, Kober S, et al. Irbesartan results in more complete blockade of human adrenal AT1-receptor mediated effects than does candesartan cilexetil as evidenced by blunted plasma aldosterone levels [abstract no. P3.225]. J Hypertens 2000 Jun; 18 Suppl. 2: S209
Kober S, Butzer R, Langguth P, et al. Extent and duration of angiotensin II antagonistic activity of irbesartan and candesartan cilexetil [abstract no. A105]. Am J Hypertension 2000 Apr; 13 (4 Pt 2): 150A
Belz GG, Butzer R, Kober S, et al. Irbesartan results in more complete blockade of human renal AT1-receptor mediated effects than does candesartan cilexetil as evidenced by higher plasma renin levels [abstract no. A106]. Am J Hypertens 2000 Apr; 13 (4 Pt 2): 151A
McIntyre M, MacFadyen RJ, Meredith PA, et al. Dose-ranging study of the angiotensin II receptor antagonist irbesartan (SR 47436/BMS-186295) on blood pressure and neurohormonal effects in salt-deplete men. J Cardiovasc Pharmacol 1996 Jul; 28(1): 101–6
Azizi M, Bissery A, Bura-Riviere A, et al. Dual renin-angiotensin system blockade restores blood pressure-renin dependency in individuals with low renin concentrations. J Hypertens 2003 Oct; 21(10): 1887–95
Lauten WB, Khan QA, Rajagopalan S, et al. Usefulness of quinapril and irbesartan to improve the anti-inflammatory response of atorvastatin and aspirin in patients with coronary artery disease. Am J Cardiol 2003 May 1; 91: 1116–9
Burnier M, Pechere-Bertschi A, Nussberger J, et al. Studies of the renal effects of angiotensin II receptor blockade: the con-founding factor of acute water loading on the action of vasoac-tive systems. Am J Kidney Dis 1995 Jul; 26(1): 108–15
Vachharajani NN, Shyu WC, Chando TJ, et al. Oral bioavailability and disposition characteristics of irbesartan, an angiotensin antagonist, in healthy volunteers. J Clin Pharmacol 1998 Aug; 38(8): 702–7
Chando TJ, Everett DW, Kahle AD, et al. Biotransformation of irbesartan in man. Drug Metab Dispos 1998 May; 26(5): 408–17
Vachharajani NN, Shyu WC, Smith RA, et al. The effects of age and gender on the pharmacokinetics of irbesartan. Br J Clin Pharmacol 1998 Dec; 46(6): 611–3
Marino MR, Langenbacher KM, Raymond RH, et al. Pharmacokinetics and pharmacodynamics of irbesartan in patients with hepatic cirrhosis. J Clin Pharmacol 1998 Apr; 38(4): 347–56
Kostis JB, Vachharajani NN, Hadjilambris OW, et al. The pharmacokinetics and pharmacodynamics of irbesartan in heart failure. J Clin Pharmacol 2001 Sep; 41(9): 935–42
Vachharajani N, Shyu WC, Mantha S, et al. Lack of food effect on the oral bioavailability of irbesartan [abstract no. 60]. J Clin Pharmacology 1997 Sep; 37(9): 872
Morsing P, Adler G, Brandt-Eliasson U, et al. Mechanistic differences of various AT1-receptor blockers in isolated vessels of different origin. Hypertension 1999 Jun; 33(6): 1406–13
Ruilope L. Human pharmacokinetic/pharmacodynamic profile of irbesartan: a new potent angiotensin II receptor antagonist. J Hypertens 1997 Dec; 15 Suppl. 7: S15–20
Perrier L, Bourrié M, Marti E, et al. In vitro N-glucuronidation of SR 474346 (BMS 186295), a new AT1 nonpeptide angiotensin II receptor antagonist, by rat, monkey and human hepatic microsomal fractions. J Pharmacol Exp Ther 1994 Oct; 271(1): 91–9
Bourrié M, Meunier V, Berger Y, et al. Role of cytochrome P-4502C9 in irbesartan oxidation by human liver microsomes. Drug Metab Dispos 1999 Feb; 27(2): 288–96
Marino MR, Vachharajani NN. Drug interactions with irbesartan. Clin Pharmacokinet 2001; 40(8): 605–14
Marino MR, Hammett JL, Ferreira I, et al. Effect of nifedipine on the steady-state pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Cardiovasc Pharmacol Ther 1998 Apr; 3(2): 111–8
Mangold B, Gielsdorf W, Marino MR. Irbesartan does not affect the steady-state pharmacodynamics and pharmacokinetics of warfarin. Eur J Clin Pharmacol 1999 Oct; 55(8): 593–8
Marino MR, Vachharajani NN, Hadjilambris OW. Irbesartan does not affect the pharmacokinetics of simvastatin in healthy subjects. J Clin Pharmacol 2000 Aug; 40(8): 875–9
Marino MR, Langenbacher KM, Mangold B, et al. Lack of drug interactions with irbesartan: a summary of five pharmacokinetic studies [abstract no. P31.096]. J Hypertens 1998 Jun; 16 Suppl. 2: S248
Kovacs SJ, Wilton JH, Blum RA. Steady state (SS) pharmacokinetics (PK) of irbesartan alone and in combination with fluconazole (F) [abstract no. PI-59]. Clin Pharmacol Ther 1999 Feb; 65(2): 132
Marino MR, Langenbacher KM, Ford NF, et al. Effect of hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of the angiotensin II blocker irbesartan. Clin Drug Invest 1997 Nov; 14(5): 383–91
Escolar M, Lopez de Ocariz A, Simon M, et al. Effects of antacids on irbesartan pharmacokinetics [abstract no. FC-9]. Meth Find Exp Clin Pharmacol 1998; 20 Suppl. A: 53
Marino MR, Hammett JL, Ferreira I, et al. Effect of irbesartan on the steady-state pharmacokinetics of digoxin in healthy male subjects [abstract no. P-278E]. ASHP Midyear Clinical Meeting 1998; 33: 2301
Lacourcière Y. A multicenter, randomized, double-blind study of the antihypertensive efficacy and tolerability of irbesartan in patients aged ≥65 years with mild to moderate hypertension. Clin Ther 2000 Oct; 22(10): 1213–24
Mimran A, Ruilope L, Kerwin L, et al. A randomised, double-blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose range of enalapril for the treatment of mild-to-moderate hypertension. J Hum Hypertens 1998 Mar; 12(3): 203–8
Coca A, Calvo C, Garcia-Puig J, et al. A multicenter, randomized, double-blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension as assessed by ambulatory blood pressure monitoring: the MAPAVEL study. Clin Ther 2002 Jan; 24(1): 126–38
Chiou K-R, Chen C-H, Ding PY-A, et al. Randomized, double-blind comparison of irbesartan and enalapril for treatment of mild to moderate hypertension. Chin Med J (Taipei) 2000 May; 63(5): 368–76
Neutel J, Germino W, Smith D, et al. The antihypertensive efficacy and safety of irbesartan compared with amlodipine for the treatment of mild-to-moderate hypertension [abstract no. D068]. Am J Hypertens 1999 Apr; 12 (4 Pt 2): 128A plus poster
Mancia G, Korlipara K, van Rossum P, et al. An ambulatory blood pressure monitoring study of the comparative antihypertensive efficacy of two angiotensin II receptor antagonists, irbesartan and valsartan. Blood Press Monit 2002 Apr; 7(2): 135–42
Kassler-Taub K, Littlejohn T, Elliott W, et al. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan, in mild-to-moderate hypertension. Irbesartan/ Losartan Study Investigators [published erratum appears in Am J Hypertension 1998 Jun; 11 (6 Pt 1): 736]. Am J Hypertens 1998; 11 (4 Pt 1): 445–53
Oparil S, Guthrie R, Lewin AJ, et al. An elective-titration study of the comparative effectiveness of two angiotensin II-receptor blockers, irbesartan and losartan: Irbesartan/Losartan Study Group Investigators. Clin Ther 1998 May–Jun; 20(3): 398–409
Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension [published erratum appears in J Clin Hypertens (Greenwich) 2001 Nov–Dec; 3 (6): 395]. J Clin Hypertens (Greenwich) 2001 Sep–Oct; 3(5): 283–91, 318
Stumpe KO, Haworth D, Hoglund C, et al. Comparison of the angiotensin II receptor antagonist irbesartan with atenolol for treatment of hypertension. Blood Press 1998 Jan; 7(1): 31–7
Fogari R, Ambrosoli S, Corradi L, et al. 24-hour blood pressure control by once-daily administration of irbesartan assessed by ambulatory blood pressure monitoring: Irbesartan Multicenter Investigators’ Group. J Hypertens. 1997 Dec; 15 (12 Pt 1): 1511–8
Pool JL, Guthrie RM, Littlejohn III TW, et al. Dose-related antihypertensive effects of irbesartan in patients with mild-to-moderate hypertension. Am J Hypertens 1998 Apr; 11 (4 Pt 1): 462–70
Guthrie R, Saini R, Herman T, et al. Efficacy and tolerability of irbesartan, an angiotensin II receptor antagonist, in primary hypertension: a double-blind, placebo-controlled, dose-titration study. Multicentre Investigators. Clin Drug Invest 1998 Mar; 15(3): 217–27
Reeves RA, Lin C-S, Kassler-Taub K, et al. Dose-related efficacy of irbesartan for hypertension: an integrated analysis. Hypertension 1998 Jun; 31(6): 1311–6
Weber M, Saini R, Kassler-Taub K, et al. Irbesartan combined with low-dose hydrochlorothiazide for mild-to-moderate hypertension [abstract no. P16.27]. J Hypertens 1998 Jun; 16 Suppl. 2: S129
Howe P, Phillips P, Saini R, et al. The antihypertensive efficacy of the combination of irbesartan and hydrochlorothiazide assessed by 24-hour ambulatory blood pressure monitoring. Clin Exper Hypertension 1999 Nov; 21(8): 1373–96
Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non-responsive to hydrochlorothiazide alone. J Clin Pharm Ther 1998 Dec; 23(6): 433–40
Weir MR, Tolchin N, Toth P, et al. Addition of hydrochlorothiazide to irbesartan produces further dose-related reductions in blood pressure within two weeks [abstract no. P16.34]. J Hypertens 1998 Jun; 16 Suppl. 2: S130
Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12 (8 Pt 1): 797–805
Larochelle P, Flack JM, Marbury TC, et al. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension: Irbesartan Multicenter Investigators. Am J Cardiol 1997 Dec 15; 80(12): 1613–5
Littlejohn III T, Saini R, Kassler-Taub K, et al. Long-term safety and antihypertensive efficacy of irbesartan: pooled results of five open-label studies. Clin Exper Hypertension 1999 Nov; 21(8): 1273–95
Raskin P, Guthrie R, Flack JM, et al. The long-term antihypertensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens 1999 Oct; 13(10): 683–7
Bays HE, Park JS, Reilly K, et al. Irbesartan safety and effectiveness; a postmarketing surveillance study [abstract no. D039]. AST investigators. Am J Hypertens 1999; 12 (4 Pt 2): 120A
Kurland L, Melhus H, Karlsson J, et al. Angiotensin converting enzyme gene polymorphism predicts blood pressure response to angiotensin II receptor type 1 antagonist treatment in hypertensive patients. J Hypertens 2001 Oct; 19: 1783–7
Kurland L, Melhus H, Karlsson J, et al. Aldosterone synthase (CYP11B2)-344 C/T polymorphism is related to antihyperten-sive response: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation Versus Atenolol (SILVHIA) trial. Am J Hypertens 2002; 15(5): 389–93
Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT). J Clin Hypertens 2002 Nov–Dec; 4(6): 393–404
Coca A, Calvo C, Sobrino J, et al. Once-daily fixed-combination irbesartan 300mg/hydrochlorothiazide 25mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther 2003 Nov; 25(11): 2849–64
Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol 1987; 10 Suppl. 6: S135–40
Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990 May 31; 322(22): 1561–6
McLenachan JM, Henderson E, Morris KI, et al. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med 1987 Sep 24; 317(13): 787–92
Villatico Campbell S, Rizzo V, Di Maio F, et al. Comparison of irbesartan versus enalapril on left ventricular mass and function in stage I–II hypertensives [abstract no. P.70]. J Hypertens 1998 Jun; 16 Suppl. 2: S18
Feraco E. Therapeutic effects of irbesartan in left ventricular hypertrophy [abstract no. DO19]. Am J Hypertens 1999 Apr; 12 (4 Pt 2): 115A
Malmqvist K, Kahan T, Edner M, et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens 2001 Jun; 19(6): 1167–76
Gaudio C, Ferri FM, Giovannini M, et al. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol 2003 Nov; 42(5): 622–8
Malmqvist K, Kahan T, Edner M, et al. Comparison of actions of irbesartan versus atenolol on cardiac repolarization in hypertensive left ventricular hypertrophy: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation Versus Atenolol (SILVHIA). Am J Cardiol 2002 Nov 15; 90(10): 1107–12
Pohl M, Cooper M, Ulrey J, et al. Safety and efficacy of irbesartan in hypertensive patients with type II diabetes and proteinuria [abstract no. D4]. Am J Hypertens 1997 Apr; 10 (Pt 2): 105
Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001 Sep 20; 345(12): 870–8
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001 Sep 20; 345(12): 851–60
Sasso FC, Carbonara O, Persico M, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: a randomized double-blind placebo-controlled crossover study. Diabetes Care 2002 Nov; 25(11): 1909–13
Rossing K, Christensen PK, Andersen S, et al. Comparative effects of irbesartan on ambulatory and office blood pressure: a substudy of ambulatory blood pressure from the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study. Diabetes Care 2003 Mar; 26(3): 569–74
Andersen S, Bröchner-Mortensen J, Parving H-H. Kidney function during and after withdrawal of long-term irbesartan treatment in patients with type 2 diabetes and microalbuminuria: Irbesartan in patients with type 2 diabetes and microalbuminuria Study Group. Diabetes Care 2003 Dec; 26(12): 3296–302
Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy: Collaborative Study Group. Ann Intern Med 2003 Apr 1; 138(7): 542–9
Palmer AJ, Annemans L, Roze S, et al. An economic evaluation of irbesartan in the treatment of patients with type 2 diabetes, hypertension and nephropathy: cost-effectiveness of Irbesartan in Diabetic Nephropathy Trial (IDNT) in the Belgian and French settings. Nephrol Dial Transplant 2003 Oct; 18(10): 2059–66
Rodby RA, Chiou C-F, Borenstein J, et al. The cost-effectiveness of irbesartan in the treatment of hypertensive patients with type 2 diabetic nephropathy. Clin Ther 2003; 25(7): 2102–19
Coyle D, Rodby RA. Economic evaluation of the use of irbesartan and amlodipine in the treatment of diabetic nephropathy in patients with hypertension in Canada. Can J Cardiol 2004 Jan; 20(1): 71–9
Koch H, Pirk O, Schöffski, et al. Treatment of hypertensive type 2 diabetics (t2d) with irbesartan (Irb) is cost-saving due to prevented or delayed end-stage renal disease [abstract no. 22]. Int J Clin Pharm and Ther 2003; 41(11): 537–8
Simon TA, Gelarden RT, Freitag SA, et al. Safety of irbesartan in the treatment of mild to moderate systemic hypertension. Am J Cardiol 1998 Jul; 82(2): 179–82
Sanofi Pharma, Bristol-Myers Squibb SNC. Aprovel 75mg tablets; Aprovel 150mg tablets; Aprovel 300mg tablets: summary of product characteristics. Paris: Sanofi Pharma, Bristol-Myers Squibb SNC, 2002 Jun
Bristol-Myers Squibb Company, Sanofi-Synthelabo. Avalide® (irbesartan-hydrochlorothiazide) tablets: prescribing information. New York: Bristol-Myers Squibb Sanofi-Synthelabo Partnership, 2002 Apr
Bristol-Myers Squibb Company, Sanofi-Synthelabo. Avapro® (irbesartan) tablets: prescribing information. New York: Bristol-Myers Squibb Sanofi-Synthelabo Partnership, 2002 Aug
Sweetman SC. Martindale: the complete drug reference. 33rd ed. London: Pharmaceutical Press, 2002
Sanofi-Synthelabo Australia Pty Limited. Product information: Karvea® (irbesartan) [online]. Available from URL: http://www.sanofi-synthelabo.co.au [Accessed 2004 Mar 23]
Doctor’s Guide. Health Canada approves Avapro (irbesartan) for the treatment of diabetic renal disease [online]. Available from URL: http://www.pslgroup.com/dg [Accessed 2004 Mar 23]
World Health Organization. The world health report 2002: risks to health 2002. Quantifying Selected Major Risks to health. Geneva: World Health Organization, 2002: 47–96
Collins R, MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Br Med Bull 1994 Apr; 50(2): 272–98
World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003 Nov; 21(11): 1983–92
Guidelines Committee. 2003 European Society of Hypertension —European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003 Jun; 21(6): 1011–53
Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [published erratum appears in JAMA 2003 Jul 9; 290(2): 197]. JAMA 2003 May 21; 289(19): 2560–72
Parati G, Pomidossi G, Albini F, et al. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987 Feb; 5(1): 93–8
Frattola A, Parati G, Cuspidi C, et al. Prognostic value of 24-hour blood pressure variability. J Hypertens 1993 Oct; 11(10): 1133–7
Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002 Mar 23; 359(9311): 995–1003
Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002 Mar 23; 359(9311): 1004–10
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Med 1997; 14 Suppl. 5: 1–85
Ritz E, Rychlik I, Locatelli F, et al. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999 Nov; 34(5): 795–808
United States Renal Data System. 2001 Annual Data Report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD, 2001. Chapter 1: 37–52
Parving HH, Andersen AR, Smidt UM, et al. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983 May 28; 1(8335): 1175–9
Mogensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. Br Med J (Clin Res Ed) 1982 Sep 11; 285(6343): 685–8
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group [published erratum appears in Br Med J 1999 Jan 2; 318 (7175): 29]. Br Med J 1998 Sep 12; 317(7160): 703–13
Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators [published erratum appears in Lancet 2000 Sep 2; 356 (9232): 860]. Lancet 2000 Jan 22; 355(9200): 253–9
Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes mellitus. Diabetes Care 2000 Apr; 23 Suppl. 2: B54–64
Salvetti A, Mattei P, Sudano I. Renal protection and antihypertensive drugs: current status. Drugs 1999 May; 57(5): 665–93
Parving HH, Tarnow L, Rossing P. Renal protection in diabetes: an emerging role for calcium antagonists. J Hypertens 1996 Nov; 14 Suppl. 4: S21–5
Toto R. Angiotensin II subtype 1 receptor blockers and renal function. Arch Intern Med 2001 Jun 25; 161(12): 1492–9
Ramsay LE, Williams B, Johnston GD, et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ 1999 Sep 4; 319(7210): 630–5
Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Disease 2000 Sep; 36(3): 646–61
Meltzer S, Leiter L, Daneman D, et al. 1998 clinical practice guidelines for the management of diabetes in Canada: Canadian Diabetes Association. CMAJ 1998; 159 Suppl. 8: 1–29
Arauz-Pacheco C, Parrott MA, Raskin P, et al. Treatment of hypertension in adults with diabetes. American Diabetes Association. Diabetes Care 2003 Jan; 26 Suppl. 1: S80–2
Joint British recommendations on prevention of coronary heart disease in clinical practice: summary. British Cardiac Society, British Hyperlipidaemia Association, British Hypertension Society, British Diabetic Association. BMJ 2000 Mar 11; 320(7236): 705–8
Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998 Jul 18; 352(9123): 213–9
Ruilope L. RAS blockade: new possibilities in the treatment of complications of diabetes. Heart 2000 Sep; 84 Suppl. 1: i32–4, discussion i50
Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy: the Collaborative Study Group [published erratum appears in N Engl J Med 1993 Jan 13; 330 (2): 152]. N Engl J Med 1993 Nov 11; 329(20): 1456–62
Viberti G, Mogensen CE, Groop LC, et al. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria: European Microalbuminuria Captopril Study Group. JAMA 1994 Jan 26; 271(4): 275–9
Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the Candesartan And Lisinopril Microalbuminuria (CALM) study. BMJ 2000 Dec 9; 321(7274): 1440–4
Agardh CD, Garcia-Puig J, Charbonnel B, et al. Greater reduction of urinary albumin excretion in hypertensive type II diabetic patients with incipient nephropathy by lisinopril than by nifedipine. J Hum Hypertens 1996 Mar; 10(3): 185–92
Lacourcière Y, Belanger A, Godin C, et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int 2000 Aug; 58(2): 762–9
Schnack C, Hoffmann W, Hopmeier P, et al. Renal and metabolic effects of 1-year treatment with ramipril or atenolol in NIDDM patients with microalbuminuria. Diabetologia 1996 Dec; 39(12): 1611–6
Nielsen FS, Rossing P, Gall MA, et al. Long-term effect of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes 1997 Jul; 46(7): 1182–8
Bakris GL, Copley JB, Vicknair N, et al. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int 1996 Nov; 50(5): 1641–50
Ravid M, Lang R, Rachmani R, et al. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus: a 7-year follow-up study. Arch Intern Med 1996 Feb 12; 156(3): 286–9
Gansevoort RT, Sluiter WJ, Hemmelder MH, et al. Antiproteinuric effect of blood-pressure-lowering agents: a meta-analysis of comparative trials. Nephrol Dial Transplant 1995 Nov; 10(11): 1963–74
Kasiske BL, Kalil RS, Ma JZ, et al. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Ann Intern Med 1993 Jan 15; 118(2): 129–38
Weidmann P, Schneider M, Bohlen L. Therapeutic efficacy of different antihypertensive drugs in human diabetic nephropathy: an updated meta-analysis. Nephrol Dial Transplant 1995 Oct; 10 Suppl 9: 39–45
Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001 Sep 20; 345(12): 861–9
Muirhead N, Feagan BF, Mahon J, et al. The effects of valsartan and captopril on reducing microalbuminuria in patients with type 2 diabetes mellitus: a placebo-controlled trial. Curr Ther Res 1999 Dec; 60: 650–60
Viberti G, Wheeldon NM, Microalbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood-pressure-independent effect. Circulation 2002; 106: 672–8
Molitch ME, De Fronzo RA, Franz MJ, et al. Diabetic nephropathy: American Diabetes Association. Diabetes Care 2003 Jan; 26 Suppl. 1: S94–8
Working Party of the International Diabetes Federation (European Region). Hypertension in people with type 2 diabetes: knowledge-based diabetes-specific guidelines. Diabet Med 2003 Dec; 20(12): 972–87
Pylypchuk GB. ACE inhibitor-versus angiotensin II blocker-induced cough and angioedema. Ann Pharmacother 1998 Oct; 32(10): 1060–6
Mazzolai L, Burnier M. Comparative safety and tolerability of angiotensin II receptor antagonists. Drug Saf 1999 Jul; 21(1): 23–33
Lacourcière Y, Brunner H, Irwin R, et al. Effects of modulators of the renin-angiotensin-aldosterone system on cough: Losartan Cough Study Group. J Hypertens 1994 Dec; 12(12): 1387–93
Benz J, Oshrain C, Henry D, et al. Valsartan, a new angiotensin II receptor antagonist: a double-blind study comparing the incidence of cough with lisinopril and hydrochlorothiazide. J Clin Pharmacol 1997 Feb; 37(2): 101–7
Rossing K, Christensen PK, Jensen BR, et al. Dual blockade of the renin-angiotensin system in diabetic nephropathy: a randomized double-blind crossover study. Diabetes Care 2002 Jan; 25(1): 95–100
Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant 2002 Jun; 17(6): 1019–24
Jacobsen P, Andersen S, Rossing K, et al. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int 2003 May; 63(5): 1874–80
Jacobsen P, Andersen S, Jensen BR, et al. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol 2003; 14: 992–9
Rossing K, Jacobsen P, Pietraszek L, et al. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy. Diabetes Care 2003; 26(8): 2268–74
Cannon CP. Innovative directions in th future role of antiplatelet agents. In: Califf RM, Topol EJ, Mehta SR et al. Therapeutic Challenges in the Treatment of Cardiovascular Disease: issue and answers [online]. Available from URL: http://www.medscape.com/viewprogram/2891_pnt [Accessed 2004 Mar 9]
Bannerjee P, Clark AL, Cleland JGF. Diastolic heart failure: a difficult problem in the elderly. Am J Geriatr Cardiol 2004; 13(1): 16–21
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: N.H. Andersen, Department of Diabetes and Endocrinology, Aarhus Kommunehospital, Aarhus, Denmark; M. Burnier, Polyclinique Medicale Universitaire, Lausanne, Switzerland; A. Coca, Department of Hypertension, Hospital Clinic, University of Barcelona, Barcelona, Spain; M.E. Cooper, Danielle Alberti Memorial Centre for Diabetes Complications, Heart Research Institute, Melbourne, Australia; G. Jerums, Endocrine Unit, Austin Hospital, Heidelberg, Australia; C.E. Mogensen, Department of Diabetes and Endocrinology, Aarhus Kommunehospital, Aarhus, Denmark; L.M. Ruilope, Hypertension Unit, Hospital 12 de Octubre, Madrid, Spain; A. Salvetti, Department of Medicine, University of Pisa, Pisa, Italy; G.L. Schwartz, Division of Hypertension, Mayo Clinic, Rochester, Minnesota, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on irbesartan, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘irbesartan’ and ‘diabetic nephropath*’. EMBASE search terms were ‘irbesartan’ and (‘diabetic nephropath*’ or ‘hypertension’). AdisBase search terms were ‘irbesartan’ or ‘BMS-186295’ or ‘SR 47436’ and (‘diabetic-nephropathies’ or ‘hypertension’). Searches were last updated 25 March 2004.
Selection: Studies in patients with hypertension or those with hypertension and type 2 diabetes mellitus with microalbuminuria or diabetic nephropathy who received irbesartan. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Diabetic nephropathy, hypertension, irbesartan, pharmacodynamics, pharmacokinetics, therapeutic use, type 2 diabetes mellitus.
Rights and permissions
About this article
Cite this article
Croom, K.F., Curran, M.P., Goa, K.L. et al. Irbesartan. Drugs 64, 999–1028 (2004). https://doi.org/10.2165/00003495-200464090-00011
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464090-00011