Abstract
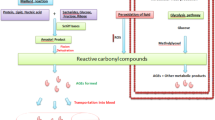
Advanced glycation endproducts (AGEs) are formed by a reaction between reducing sugars and biological amines. Because of their marked stability, glycated proteins accumulate slowly over a person’s lifespan, and can contribute to age-associated structural and physiological changes in the cardiovascular system such as increased vascular and myocardial stiffness, endothelial dysfunction, altered vascular injury responses and atherosclerotic plaque formation. The mechanisms by which AGEs affect the cardiovascular system include collagen crosslinking, alteration of low-density lipoprotein molecules and impairment of cellular nitric oxide signalling through their interaction with AGE receptors (RAGEs). Thus, the accumulation of AGEs may help to explain the increased cardiac risk associated with aging as well as diabetes mellitus and hypertension, two conditions that accelerate and enhance AGE formation.
A variety of new pharmacological approaches are being developed to reduce the pathophysiological impact of AGEs. These agents can prevent AGE and AGE crosslink formation, break pre-existing AGE crosslinks, and block the interaction between AGEs and RAGEs. Such agents have been shown to reduce vascular and myocardial stiffness, inhibit atherosclerotic plaque formation and improve endothelial function in animal models. Improvement in vascular compliance has also been demonstrated with AGE crosslink breakers in clinical trials. These studies offer promise to reduce the cardiac risk associated with isolated systolic hyperten-sion, diastolic dysfunction and diabetes.


Similar content being viewed by others
References
Maillard L, Gautier M. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Seances Acad Sci III 1912; 154: 66–8
Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001; 56: 1–21
Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A 1984; 81: 583–7
Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988; 318: 1315–21
Cerami A. Hypothesis: glucose as a mediator of aging. J Am Geriatr Soc 1985; 33: 626–34
Koenig RJ, Peterson CM, Jones RL, et al. Correlation of glucose regulation and hemoglobin Alc in diabetes mellitus. N Engl J Med 1976; 295: 417–20
Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000; 275: 39027–31
De Souza RR. Aging of myocardial collagen. Biogerontology 2002; 3: 325–35
Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 2001; 122: 735–55
Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol 2001; 85: 746–53
Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest 1981; 67: 1630–5
Brownlee M, Pongor S, Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen: role in the in situ formation of immune complexes. J Exp Med 1983; 158: 1739–44
Stern D, Du YS, Fang YS, et al. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev 2002; 54: 1615–25
Wendt T, Bucciarelli L, Qu W, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep 2002; 4: 228–37
Sims TJ, Rasmussen LM, Oxlund H, et al. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 1996; 39: 946–51
Bishop JE, Lindahl G. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res 1999; 42: 27–44
Weiss MF, Erhard P, Kader-Attia FA, et al. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int 2000; 57: 2571–85
Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 1994; 269: 9889–97
Vlassara H, Striker LJ, Teichberg S, et al. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A 1994; 91: 11704–8
Bucala R, Mitchell R, Arnold K, et al. Identification of the major site of apolipoprotein B modification by advanced glycosylation end products blocking uptake by the low density lipoprotein receptor. J Biol Chem 1995; 270: 10828–32
Herrmann KL, McCulloch AD, Omens JH. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am J Physiol Heart Circ Physiol 2003; 284: H1277–84
Petrova R, Yamamoto Y, Muraki K, et al. Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol 2002; 34: 1425–31
Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol (Paris) 2001; 49: 310–25
Brownlee M, Vlassara H, Kooney A, et al. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986; 232: 1629–32
Monnier VM, Vishwanath V, Frank KE, et al. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 1986; 314: 403–8
Winlove CP, Parker KH, Avery NC, et al. Interactions of elastin and aorta with sugars in vitro and their effects on biochemical and physical properties. Diabetologia 1996; 39: 1131–9
MacKenna DA, Omens JH, McCulloch AD, et al. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am J Physiol 1994; 266: H1007–18
Avendano GF, Agarwal RK, Bashey RI, et al. Effects of glucose intolerance on myocardial function and collagenlinked glycation. Diabetes 1999; 48: 1443–7
Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation products on collagen covalently trap low-density lipoprotein. Diabetes 1985; 34: 938–41
Bucala R, Makita Z, Koschinsky T, et al. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A 1993; 90: 6434–8
Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88: 1785–92
Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci 2001; 6: D1151–60
Thornalley PJ. Cell activation by glycated proteins: AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998; 44: 1013–23
Schmidt AM, Mora R, Cao R, et al. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem 1994; 269: 9882–8
Throckmorton DC, Brogden AP, Min B, et al. PDGF and TGF-beta mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int 1995; 48: 111–7
Schmidt AM, Stern D. Atherosclerosis and diabetes: the RAGE connection. Curr Atheroscler Rep 2000; 2: 430–6
Twigg SM, Chen MM, Joly AH, et al. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology 2001; 142: 1760–9
Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–47
American Heart Association. 2003 heart and stroke statistical update. Dallas (TX): American Heart Association, 2002
Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 1993; 73: 413–67
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Pt I: aging arteries: a ‘set up’ for vascular disease. Circulation 2003; 107: 139–46
McVeigh G, Bratteli C, Morgan D, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension 1999; 33: 1392–8
Nichols WW, O’Rourke MF, editors. McDonald’s blood flow in arteries: theoretical, experimental and clinical principles. 4th ed. London: Edward Arnold, 1998: 398–420
Van Bortel LM, Struijker-Boudier HA, Safar ME. Pulse pressure, arterial stiffness, and drug treatment of hypertension. Hypertension 2001; 38: 914–21
Dart A, Kingwell B. Pulse pressure: a review of mechanisms and clinical relevance. J Am Coll Cardiol 2001; 37: 975–84
The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157: 2413–46
Roman M, Ganau A, Saba P, et al. Impact of arterial stiffening on left ventricular structure. Hypertension 2000; 36: 489–94
Kass D. Age-related changes in ventricular-arterial coupling: pathophysiologic implications. Heart Fail Rev 2002; 7: 51–62
Nussbacher A, Gerstenblith G, O’Connor FC, et al. Hemodynamic effects of unloading the old heart. Am J Physiol 1999; 277: H1863–71
Chen C-H, Nakayama M, Talbot M, et al. Verapamil acutely reduces ventricular-vascular stiffness and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol 1999; 33: 1602–9
Vaitkevicius P, Fleg J, Engel J, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993; 88: 1456–62
Saeki A, Recchia F, Kass D. Systolic flow augmentation in hearts ejecting into a model of stiff aging vasculature: influence on myocardial perfusion-demand balance. Circ Res 1995; 76: 132–41
Sesso H, Stampfer M, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension 2000; 36: 801–7
Blacher J, Staessen J, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000; 160: 1085–9
Khattar R, Swales J, Dore C, et al. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation 2001; 104: 783–9
Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a trench male population. Hypertension 1997; 30: 1410–5
Chae C, Pfeffer MA, Glynn R, et al. Increased pulse pressure and risk of heart failure in the elderly. JAMA 1999; 281: 634–9
Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation 1999; 100: 354–60
Mitchell GF, Pfeffer MA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. Circulation 1997; 96: 4254–60
O’Donnell C, Ridker P, Glynn R, et al. Hypertension and borderline isolated systolic hypertension increase risks of cardiovascular disease and mortality in male physicians. Circulation 1997; 95: 1132–7
Vaccarino V, Holford T, Krumholz H. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol 2000; 36: 130–8
Vaccarino V, Berger A, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001; 88: 980–6
Kostis J, Lawrence-Nelson J, Ranjan R, et al. Association of increased pulse pressure with the development of heart failure in SHEP: Systolic Hypertension in the Elderly (SHEP) Cooperative Research Group. Am J Hypertens 2001; 14: 798–803
Domanski M, Davis B, Pfeffer M, et al. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension 1999; 34: 375–80
Meaume S, Benetos A, Henry O, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol 2001; 21: 2046–50
Safar M, London G. Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertens 2000; 18: 1527–35
Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103: 1245–9
Darne B, Girerd X, Safar M, et al. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989; 13: 392–400
Hansson L, Lindholm L, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999; 354: 1751–6
Hansson L, Zanchetti A, Carruthers S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351: 1755–62
Staessen J, Gasowski J, Wang J, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: metaanalysis of outcome trials. Lancet 2000; 355: 865–72
Cushman W, Materson B, Williams D, et al. Pulse pressure changes with six classes of antihypertensive agents in a randomized, controlled trial. Hypertension 2001; 38: 953–7
Safar M, Rudnichi A, Asmar R. Drug treatment of hypertension: the reduction of pulse pressure does not necessarily parallel that of systolic and diastolic blood pressure. J Hypertens 2000; 18: 1159–63
Leipzig R, Cumming R, Tinetti M. Drugs and falls in older people: a systematic reviews and meta-analysis. II: cardiac and analgesic drugs. J Am Geriatr Soc 1999; 47: 40–50
Millar J, Lever A. Implications of pulse pressure as a predictor of cardiac risk in patients with hypertension. Hypertension 2000; 36: 907–11
Lakatta EG, Yin FC. Myocardial aging: functional alterations and related cellular mechanisms. Am J Physiol 1982; 242(6): H927–41
Gerstenblith G, Frederiksen J, Yin FC, et al. Echocardiographic assessment of a normal adult aging population. Circulation 1977; 56: 273–8
Harrison TR, Dixon K, Russell RO, et al. The relation of age to the duration of contraction, ejection, and relaxation of the normal human heart. Am Heart J 1999; 67: 189–99
Schulman SP, Lakatta EG, Fleg JL, et al. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol 1992; 263: H1932–8
Bonow R, Vitale D, Bacharach S. Effect of aging on asynchronous left ventricular regional function and global ventricular filling in normal human subjects. J Am Coll Cardiol 1988; 24: 471–6
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Pt II: the aging heart in health: links to heart disease. Circulation 2003; 107: 346–54
Redfield MM, Jacobsen SJ, Burnett Jr JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202
NairVM,Tekin UN, Khan IA, et al. Worsening of left ventricular diastolic dysfunction during exercise causes decreased exercise tolerance in hypertension. Clin Cardiol 2000 Sep 2000; 23: 660–4
Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 1991; 17: 1065–72
Kitzman DW. Diastolic heart failure in the elderly. Heart Fail Rev 2002; 7: 17–27
Dodek A, Kassebaum DG, Bristow JD. Pulmonary edema in coronary-artery disease without cardiomegaly: paradox of the stiff heart. N Engl J Med 1972; 286: 1347–50
Aronow WS, Ahn C, Kronzon I. Prognosis of congestive heart failure in elderly patients with normal versus abnormal left ventricular systolic function associated with coronary artery disease. Am J Cardiol 1990; 66: 1257–9
Chen HH, Lainchbury JG, Senni M, et al. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail 2002; 8: 279–87
Aurigemma GP, Gottdiener JS, Shemanski L, et al. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol 2001; 37: 1042–8
Corman B, Duriez M, Poitevin P, et al. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci U S A 1998; 95: 1301–6
Quyyumi A. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 1998; 105: 32S–9S
Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 1993; 88: 77–81
Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 1995; 91: 1981–7
Gerhard M, Roddy M, Creager S, et al. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 1996; 27: 849–53
Yasue H, Matsuyama K, Okumura K, et al. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment: possible role of early coronary atherosclerosis. Circulation 1990; 81: 482–90
Vlassara H, Fuh H, Makita Z, et al. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A 1992; 89: 12043–7
Rojas A, Romay S, Gonzalez D, et al. Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circ Res 2000; 86: E50–4
Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Pt III: cellular and molecular clues to heart and arterial aging. Circulation 2003; 107: 490–7
Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med 2002; 251: 87–101
Airaksinen KE, Salmela PI, Linnaluoto MK, et al. Diminished arterial elasticity in diabetes: association with fluorescent advanced glycosylation end products in collagen. Cardiovasc Res 1993; 27: 942–5
Cantini C, Kieffer P, Corman B, et al. Aminoguanidine and aortic wall mechanics, structure, and composition in aged rats. Hypertension 2001; 38: 943–8
Huijberts MS, Wolffenbuttel BH, Boudier HA, et al. Aminoguanidine treatment increases elasticity and decreases fluid filtration of large arteries from diabetic rats. J Clin Invest 1993; 92: 1407–11
Panagiotopoulos S, O’Brien RC, Bucala R, et al. Aminoguanidine has an anti-atherogenic effect in the cholesterolfed rabbit. Atherosclerosis 1998; 136: 125–31
Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine. Biochem Biophys Res Commun 1996; 220: 113–9
Booth AA, Khalifah RG, Todd P, et al. Invitrokinetic studies of formation of antigenic advanced glycation end products (AGEs): novel inhibition of post-Amadori glycation pathways. J Biol Chem 1997; 272: 5430–7
Miyata T, Ueda Y, Yamada Y, et al. Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product: carbonyl stress in uremia. J Am Soc Nephrol 1998; 9: 2349–56
Alderson NL, Chachich ME, Youssef NN, et al. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int 2003; 63: 2123–33
Stitt A, Gardiner TA, Alderson NL, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes 2002; 51: 2826–32
Mizutani K, Ikeda K, Tsuda K, et al. Inhibitor for advanced glycation end products formation attenuates hypertension and oxidative damage in genetic hypertensive rats. J Hypertens 2002; 20: 1607–14
Miyata T, Ishikawa S, Asahi K, et al. 2-Isopropylidenehydrazono-4-oxo-thiazolidin-5-ylacetanilide (OPB-9195) treatment inhibits the development of intimai thickening after balloon injury of rat carotid artery: role of glycoxidation and lipoxidation reactions in vascular tissue damage. FEBS Lett 1999; 445: 202–6
Vasan S, Zhang X, Kapurniotu A, et al. An agent cleaving glucose-derived protein crosslinks invitroand invivo. Nature 1996; 382: 275–8
Cooper ME, Thallas V, Forbes J, et al. The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia 2000; 43: 660–4
Wolffenbuttel B, Boulanger C, Crijns F, et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A 1998; 95: 4630–4
Candido R, Forbes JM, Thomas MC, et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 2003; 92: 785–92
Asif M, Egan J, Vasan S, et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A 2000; 97: 2809–13
Vaitkevicius P, Lane M, Spurgeon H, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A 2001; 98: 1171–5
Figarola JL, Scott S, Loera S, et al. LR-90 a new advanced glycation endproduct inhibitor prevents progression of diabetic nephropathy in streptozotocin-diabetic rats. Diabetologia 2003; 46: 1140–52
Kass D, Shapiro E, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2001; 104: 1464–70
Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein Enull mice. Circulation 2002; 106: 2827–35
Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998; 4: 1025–31
Kislinger T, Tanji N, Wendt T, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 2001; 21: 905–10
Zhou Z, Wang K, Penn MS, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation 2003; 107: 2238–43
Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 2003; 111: 959–72
Acknowledgements
The authors have no potential conflicts of interest to declare. Dr Zieman is an E. Cowles Andrus and a Williams Scholar and is supported by grant funds from the Association of Subspecialty Professors/Society of Geriatric Cardiology and the National Heart Blood and Lung Institute (1K23HL73059-01). Dr Kass is partially supported by a grant from the National Institute on Aging (AG-18324).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zieman, S.J., Kass, D.A. Advanced Glycation Endproduct Crosslinking in the Cardiovascular System. Drugs 64, 459–470 (2004). https://doi.org/10.2165/00003495-200464050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464050-00001