Abstract
It has been postulated, consistent with the ubiquitous presence of glutamatergic neurons in the brain, that defects in glutamatergic neurotransmission are associated with many human neurological and psychiatric disorders. This review evaluates the possible application of ligands acting on glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) and kainate (KA) receptors to minimise the pathology and/or symptoms of various diseases.
Glutamate activation of AMPA receptors is thought to mediate most fast synaptic neurotransmission in the brain, while transmission via KA receptors contributes only a minor component. Variants of the protein subunits forming these receptors greatly extend the pharmacological and electrophysiological properties of AMPA/KA receptors. Disease and drug use can differentially affect the expression of the subunits and their variants.
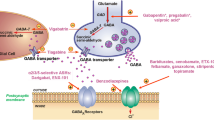
Ligands bind to AMPA receptors by competing with glutamate at the glutamate binding site, or non-competitively at other sites on the proteins (allosteric modulators). Ligands showing selective competitive antagonist actions at the AMPA/ KA class of glutamate receptors were first reported in 1988, and the systemically active antagonist 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline (NBQX) was first shown to have useful therapeutic effects on animal models of neurological diseases in 1990. Since then, newer antagonists with increased potency, higher specificity, increased water solubility, and a longer duration of action in vivo have been developed. Negative allosteric modulators such as the prototype GYKI-52466 also block AMPA receptors but have little action at KA receptors. Positive allosteric modulators enhance glutamatergic neurotransmission at AMPA receptors. Polyamines and adamantane derivatives bind within the ion channel of calcium-permeable AMPA receptors. The latest developments include ligands selective for KA receptors containing Glu-R5 subunits.
Evidence for advantages of AMPA receptor antagonists over N-methyl-D-aspartate (NMDA) receptor antagonists for symptomatic treatment of neurological and psychiatric conditions, and for minimising neuronal loss occurring after acute neurological diseases, such as physical trauma, ischaemia or status epilepticus, have been shown in animal models. However, as yet AMPA receptor antagonists have not been shown to be effective in clinical trials.
On the other hand, a limited number of clinical trials have been reported for AMPA receptor ligands that enhance glutamatergic neurotransmission by extending the ion channel opening time (positive allosteric modulators). These acute studies demonstrate enhanced memory capability in both young and aged humans, without any apparent serious adverse effects. The use of these allosteric modulators as antipsychotic drugs is also possible. However, the long term use of both direct agonists and positive allosteric modulators must be approached with considerable caution because of potential adverse effects.

Similar content being viewed by others
References
Hayashi T, Umemori H, Mishina M, et al. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature 1999; 397: 72–6
Wang Y, Small DL, Stanimirovic DB, et al. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 1997; 389: 502–4
Kawai F, Sterling P. AMPA receptor activates a G-protein that suppresses a cGMP-gated current. JNeurosci 1999; 19: 2954–9
Cunha RA, Malva JO, Ribeiro JA. Kainate receptors coupled to Gi/Go proteins in the rat hippocampus. Mol Pharmacol 1999; 56: 429–33
Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 1998; 20: 1211–18
Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res 1994; 100: 47–51
Whetsell WO. Current concepts of excitotoxicity. J Neuropathol Exp Neurol 1996; 55: 1–13
Cebers G, Zhivotovsky B, Ankarcrona M, et al. AMPA neurotoxicity in cultured cerebellar granule neurons: mode of cell death. Brain Res Bull 1997; 43: 393–403
Kovacs AD, Szabo G. GYKI 53665, a 2,3-benzodiazepine, non-competitively protects cultured neurones against AMPA toxicity. Eur J Pharmacol 1997; 331: 93–6
Larm JA, Cheung NS, Beart PM. Apoptosis induced via AMPA-selective glutamate receptors in cultured murine cortical neurons. J Neurochem 1997; 69: 617–22
Liu XH, Wang P, Barks JD. The non-competitive AMPA antagonist LY 300168 (GYKI 53655) attenuates AMPA-induced hippocampal injury in neonatal rodents. Neurosci Lett 1997; 235: 93–7
Moudy AM, Yamada KA, Rothman SM. Rapid desensitization determines the pharmacology of glutamate neurotoxicity. Neuropharmacology 1994; 33: 953–62
Ohno K, Okada M, Tsutsumi R, et al. The AMPA-receptor antagonist YM9OK reduces AMPA receptor-mediated excitotoxicity in rat hippocampal cultures. Jpn J Pharmacol 1998; 76: 105–8
Cheung NS, Carroll FY, Larm JA, et al. Kainate-induced apoptosis correlates with c-Jun activation in cultured cerebellar granule cells. J Neurosci Res 1998; 52: 69–82
Cheung NS, Pascoe CJ, Giardina SF, et al. Micromolar L-glutamate induces extensive apoptosis in an apoptotic-necrotic continuum of insult-dependent, excitotoxic injury in cultured cortical neurones. Neuropharmacology 1998; 37: 1419–29
Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: further evidence for an apoptosis-necrosis continuum. J Comp Neurol 1997; 378: 88–104
Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta 1998; 1366: 151–65
Barnard EA. Ionotropic glutamate receptors: new types and new concepts. Trends Pharmacol Sci 1997; 18: 141–8
Bettler B, Mulle C. AMPA and kainate receptors. Neuropharmacology 1995; 34: 123–39
Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 1998; 37: 1187–204
Borges K, Dingledine R. AMPA receptors: molecular and functional diversity. Prog Brain Res 1998; 116: 153–70
Chittaiallu R, Braithwaite SP, Vernon RJ, et al. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci 1999; 20: 26–35
Dev KK, Henley JM. The reguation of AMPA receptor-binding sites. Mol Neurobiol 1998; 17: 33.58
Fletcher EJ, Lodge D. New developments in the molecular pharmacology of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors. Pharmacol Ther 1996; 70: 65–89
Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci 1994; 17: 31–108
Lerma J, Morales M, Vicente MA, et al. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci 1997; 20: 9–12
Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol 1998; 54: 369–415
Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol 1998; 54: 581–618
Wenthold RJ, Roche KW. The organization and regulation of non-NMDA receptors in neurons. Prog Brain Res 1998; 116: 133–52
Seeburg EH. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci 1993; 16: 359–65
Dingledine R, Borges K, Bowie D, et al. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61
Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci 1997; 17: 2713–21
Jones KA, Wilding TJ, Huettner JE, et al. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology 1997; 36: 853–63
Zhou LM, Gu ZQ, Costa AM, et al. (2S,4R)-4-methylglutamic acid (SYM 2081): a selective, high-affinity ligand for kainate receptors. J Pharmacol Exp Ther 1997; 280: 422–7
Small B, Thomas J, Kemp M, et al. LY339434, a GluR5 kainate receptor agonist. Neuropharmacology 1998; 37: 1261–7
Toms NJ, Reid ME, Phillips W, et al. A novel kainate receptor ligand [3H]-(2S,4R)-4-methylglutamate: pharmacological characterization in rabbit brain membranes. Neuropharmacology 1997; 36: 1483–8
Donevan SD, Rogawski MA. Allosteric regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors by thiocyanate and cyclothiazide at a common modulatory site distinct from that of 2,3-benzodiazepines. Neuroscience 1998; 87: 615–29
Mano I, Teichberg VI. A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. Neuroreport 1998; 9: 327–31
Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science 1998; 280: 1596–9
Wenthold RJ, Yokotani N, Doi K, et al. Immunochemical characterization of the non-NMDA glutamate receptor using sub-unit-specific antibodies: evidence for a hetero-oligomeric structure in rat brain. J Biol Chem 1992; 267: 501–7
Archibald K, Perry MJ, Molnár E, et al. Surface expression and metabolic half-life of AMPA receptors in cultured rat cere-bellar granule cells. Neuropharmacology 1998; 37: 1345–53
Leuschner WD, Hoch W. Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their N-terminal domains. J Biol Chem 1999; 274: 16907–16
Schiffer HH, Swanson, GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron 1997; 19: 1141–6
Sommer B, Keinanen K, Verdoorn TA, et al. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science 1990; 249: 1580–5
Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci 1996; 16: 6634–47
Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Rev 1998; 26: 217–29
Liu Y, Samuel CE. Editing of glutamate receptor subunit B pre-mRNA by splice-site variants of interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR 1. J Biol Chem 1999; 274: 5070–7
Köhr G, Melcher T, Seeburg PH. Candidate editases for GluR channels in single neurons of rat hippocampus and cerebellum. Neuropharmacology 1998; 37: 1411–7
Geiger JRP, Melcher T, Koh D-S, et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 1995; 15: 193–204
Washburn MS, Nurnberger M, Zhang S, et al. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci 1997; 17: 9393–406
Burnashev N; Monyer H; Seeburg PH; Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 1992; 8: 189–98
Brorson JR, Zhang Z, Vandenberghe W. Ca2+ permeation of AMPA receptors in cerebellar neurons expressing Glu receptor 2. J Neurosci 1999; 19: 9149–59
Lomeli H, Mosbacher J, Melcher T, et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 1994; 266: 1709–13
Bernard A, Ferhat L, Dessi F, et al. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci 1999; 11: 604–16
Mulle C, Sailer A, Perez-Otano I, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 1998; 392: 601–5
Ben-Ari Y. Linibic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 1985; 14: 375–403
Bureau I, Bischoff S, Heinemann SF, et al. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci 1999; 19: 653–63
Kask K, Zamanillo D, Rozov A, et al. The AMPA receptor subunit GluR-B in its Q/R site-unedited form is not essential for brain development and function. Proc Natl Acad Sci U S A 1998; 95: 13777–82
Jia Z, Agopyan N, Miu P, et al. Enhanced LTP in mice deficient in the AMPA receptor GluR 2. Neuron 1996; 17: 945–56
Mainen ZF, Jia Z, Roder J, et al. Use-dependent AMPA receptor block in mice lacking GluR2 suggests postsynaptic site for LTP expression. Nature Neurosci 1998; 1: 579–86
Brusa R, Zimmermann F, Koh D-S, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 1995; 270: 1677–80
Feldmeyer D, Kask K, Brusa R, et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci 1999; 2: 57–64
Bliss TVP, Collingridge GL. A synaptic model of memory long term potentiation in the hippocampus. Nature 1993; 361: 31–9
Benke TA, Luthi A, Isaac JTR, et al. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 1998; 393: 793–7
Debray C, Diabira D, Gaiarsa JL, et al. Contributions of AMPA and GAB Aa receptors to the induction of NMDAR-dependent LTP in CA 1. Neurosci Lett 1997; 238: 119–22
Grover LM. Evidence for postsynaptic induction and expression of NMDA receptor independent LTP. J Neurophysiol 1998; 79: 1167–82
Nayak A, Zastrow DJ, Lickteig R, et al. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature 1998; 394: 680–3
Zamanillo D, Sprengel R, Hvalby O, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 1999; 284: 1805–11
Gerlai R, Henderson JT, Roder JC, et al. Multiple behavioral anomalies in GluR2 mutant mice exhibiting enhanced LTP. Behav Brain Res 1998; 95: 37–45
Joo DT, Xiong Z, MacDonald JF, et al. Blockade of glutamate receptors and barbiturate anesthesia: increased sensitivity to pentobarbital-induced anesthesia despite reduced inhibition of AMPA receptors in GluR2 null mutant mice. Anesthesiology 1999; 91: 1329–41
Aronica EM, Gorter JA, Grooms S, et al. Aurintricarboxylic acid prevents GluR2 mRNA down-regulation and delayed neurodegeneration in hippocampal CA1 neurons of gerbil after global ischemia. Proc Natl Acad Sci U S A 1998; 95: 7115–20
Gorter JA, Petrozzino JJ, Aronica EM, et al. Global ischemia induces downregulation of GluR2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA 1 neurons of gerbil. J Neurosci 1997; 16: 6179–88
Pellegrini-Giampietro DE, Gorter JA, Bennett MVL, et al. The GluR2 (GluR-B) hypothesis — Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci 1997; 20: 464–70
Sailer A, Swanson GT, Perez-Otano I. Generation and analysis of GluR5(Q636R) kainate receptor mutant mice. J Neurosci 1999; 19: 8757–64
Melcher T, König N, Berger T, et al. Q/R site unedited GluRB mRNA is expressed in the rat CNS at early embyronic stages [abstract]. Soc Neurosci Abstr 1997; 478.26
Bi X, Standley S, Baudry M. Posttranslational regulation of ionotropic glutamate receptors and synaptic plasticity. Int Rev Neurobiol 1998; 42: 227–84
Lynch G. Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem 1998; 70: 82–100
Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience 1997; 78: 361–71
Roche KW, O’Brien RJ, Mammen AL, et al. Characterization of multiple phosphorylation sites on the AMPA receptor GluRl subunit. Neuron 1996; 16: 1179–88
Barria A, Muller D, Derkach V, et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 1997; 276: 2042–5
Mammen AL, Kameyama K, Roche KW, et al. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 1997; 272: 32528–33
Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of AMPA receptors. J Neurosci 1999; 19: 4748–54
Soderling TR. Modulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Neurochem Int 1996; 28: 359–61
Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem 1999; 73: 1765–8
Yan Z, Hsieh-Wilson L, Feng J, et al. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci 1999; 2: 13–7
Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol (Lond) 1997; 503 (Pt 3): 513–31
Raymond LA, Backstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by c-AMP-dependent protein kinase. Nature 1993; 361: 637–41
Everts I, Villmann C, Hollmann M. N-Glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol 1997; 52: 861–73
Clark RA, Gurd JW, Bissoon N, et al. Identification of lectin-purified neural glycoproteins, GPs 180, 116, and 110, with NMDA and AMPA receptor subunits: conservation of glycosylation at the synapse. J Neurochem 1998; 70: 2594–605
Hullebroeck MF, Hampson DR. Characterization of the oligo-saccharide side chains on kainate binding proteins and AMPA receptors. Brain Res 1992; 590: 187–92
Kawamoto S, Hattori S, Sakimura K, et al. N-linked glycosylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propio-nate (AMPA)-selective glutamate receptor channel α2 subunit is essential for the acquisition of ligand-binding activity. J Neurochem 1995; 64: 1258–66
Hall RA, Hansen A, Andersen PH, et al. Surface expression of the AMPA receptor subunits GluRl, GluR2, and GluR4 in stably transfected baby hamster kidney cells. J Neurochem 1997; 68: 625–30
Muβhoff U, Madeja M, Bloms P, et al. Tunicamycin-induced inhibition of functional expression of glutamate receptors in Xenopus oocytes. Neurosci Lett 1992; 147: 163–6
Standley S, Tocco G, Wagle N, et al. High and low-affinity α-[H3]amino-3-hydroxy-5-methylisoxazole 4-propionic acid ([H3]AMPA) binding sites represent immature and mature forms of AMPA receptors and are composed of differentially glycosylated subunits. J Neurochem 1998; 70: 2434–45
Everts I, Petroski R, Kizelsztein P, et al. Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci 1999; 19: 916–27
Partin KM, Patneau DK, Winters CA, et al. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 1993; 11: 1069–82
Yue K-T, MacDonald JF, Pekhletski R, et al. Differential effects of lectins on recombinant glutamate receptors Eur J Pharmacol 1995; 291: 229–35
Bi XN, Chen J, Dang SD, et al. Characterization of calpain-mediated proteolysis of GluRl subunits of α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in rat brain. J Neurochem 1997; 68: 1484–94
Musleh W, Bi XN, Tocco G, et al. Glycine-induced long-term potentiation is associated with structural and functional modifications of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 1997; 94: 9451–6
Bi X, Chen J, Baudry M. Developmental changes in calpain activity, GluR 1 receptors and in the effect of kainic acid treatment in rat brain. Neuroscience 1997; 81: 1123–35
Pickering DS, Taverna FA, Salter MW, et al. Palmitoylation of the GluR6 kainate receptor. Proc Natl Acad Sci U S A 1995; 92: 12090–4
Chabot C, Gagne J, Giguere C, et al. Bidirectional modulation of AMPA receptor properties by exogenous phospholipase A2 in the hippocampus. Hippocampus 1998; 8: 299–309
Dev KK, Honoré T, Henley JM. Different effects of phospholipase A2 on agonist binding to hippocampal, cortical and recombinant homomeric α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Neurosci Lett 1998; 246: 25–8
Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci 1997; 17: 58–69
Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer’s disease, Huntington’s disease and schizophrenia. Brain Res 1995; 20: 297–304
Nutt SL, Kamboj RK. Differential RNA editing efficiency of AMPA receptor subunit GluR-2 in human brain. Neuroreport 1994; 5: 1679–83
Götz T, Kraushaar U, Geiger J, et al. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci 1997; 17: 204–15
Meucci O, Miller RJ. Dissociation between the Joro spider toxin sensitivity of recombinant α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and their ability to increase intracellular calcium. Neuropharmacology 1998;37: 1431–43
Utz AL, Verdoorn TA. Recombinant AMPA receptors with low Ca2+ permeability increase intracellular Ca2+ in HEK 293 cells. Neuroreport 1997; 8: 1975–80
Baltrons MA, Garcia A. AMPA receptors are coupled to the nitric oxide cyclic GMP pathway in cerebellar astroglial cells. Eur JNeurosci 1997; 9: 2497–501
Egebjerg J, Heinemann SE. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR 6. Proc Natl Acad Sci U S A 1993; 90: 755–9
Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol (Lond) 1996; 496 (Pt 1): 165–73
Burnashev N, Zhou Z, Neher E, et al. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol (Lond) 1995; 485 (Pt 2): 403–18
Köhler M, Burnashev N, Sakmann B, et al. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron 1993; 10: 491–500
Clements JD, Lester RAJ, Tong G, et al. The time course of glutamate in the synaptic cleft. Science 1992; 258: 1498–501
Geiger JRP, Lübke J, Roth A, et al. Sub millisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 1997; 18: 1009–23
Clements JD, Feltz A, Sahara Y, et al. Activation kinetics of AMPA receptor channels reveal the number of functional agonist binding sites. J Neurosci 1998; 18: 119–27
Hausser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. J Physiol 1997; 501: 77–95
Jahn K, Butler J, Franke C. Kinetics of AMPA-type glutamate receptor channels in rat caudate-putamen neurones show a wide range of desensitization but distinct recovery characteristics. Eur J Neurosci 1998; 10: 664–72
Raman TM, Trussell LO. Concentration-jump analysis of voltage-dependent conductances activated by glutamate and kainate in neurons of the avian cochlear nucleus. Biophys J 1995; 69: 1868–79
Raman IM, Trussell LO. The mechanism of α-amino-3-hydroxy-5-methy1-4-isoxazolepropionate receptor desensitization afterremoval of glutamate. BiophysJ 1995;68: 137–46
Ranimes G, Swandulla D, Spielmanns P, et al. Interactions of GYKI 52466 and NBQX with cyclothiazide at AMPA receptors: experiments with outside-out patches and EPSCs in hippocampal neurones. Neuropharmacology 1998; 37: 1299–320
Kleppe IC, Robinson HP. Determining the activation time course of synaptic AMPA receptors from openings of colocalized NMDA receptors. Biophys J 1999; 77: 1418–27
Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol 1999; 82: 1295–302
Silver RA, Colquhoun D, Cull-Candy SG, et al. Deactivation and desensitization of non-NMDA receptors in patches and the time course of EPSCs in rat cerebellar granule cells published erratum appears in J Physiol (Lond) 1996; 496: 891] J Physiol (Lond) 1996; 493: 167–73
Takahashi M, Kovalchuk Y, Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci 1995; 15: 5693–702
Arai A, Lynch G. The waveform of synaptic transmission at hippocampal synapses is not determined by AMPA receptor desensitization. Brain Res 1998; 799: 230–34
Arai A, Lynch G. AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res 1998; 799: 235–42
Mosbacher J, Schoepfer R, Monyer H, et al. A molecular determinant for sub-millisecond desensitization in glutamate receptors. Science 1994; 266: 1059–62
Angulo MC, Lambolez B, Audinat E, et al. Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J Neurosci 1997; 17: 6685–96
Titz S, Keller BU. Rapidly deactivating AMPA receptors determine excitatory synaptic transmission to interneurons in the nucleus tractus solitarius from rat. J Neurophysiol 1997; 78: 82–91
Sahara Y, Noro N, Iida Y, et al. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J Neurosci 1997; 17: 6611–20
Paternain AV, Rodríguez-Moreno A, Villarroel A, et al. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology 1998; 37: 1249–59
Swanson GT, Heinemann SF. Heterogeneity of homomeric GluR5 receptor desensitization expressed in HEK293 cells. J. Physiol (Lond) 1998; 513: 639–46
Heckmann M, Butler J, Franke C, et al. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J 1996; 71: 1743–50
Swanson GT, Gereau RW, Green T, et al. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron 1997; 19: 913–26
Swanson GT, Green T, Heinemann SF. Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Mol Pharmacol 1998; 53: 942–9
Castillo PE, Malenka RC, and Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 1997; 388: 182–6
Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature 1997; 388: 179–82
Vignes M, Clarke VR, Parry MJ, et al. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat. Neuropharmacology 1998; 37: 1269–77
Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1998; 1: 479–86
Cossart R, Esclapez M, Hirsch JC, et al. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. NatNeurosci 1998; 1: 470–8
Clarke VR, Ballyk BA, Hoo KH, et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 1997; 389: 599–603
Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically down-regulate GABAergic inhibition in the rat hippocampus. Neuron 1997; 19: 893–901
Frerking M, Petersen CC, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc Natl Acad Sci U S A 1999; 96: 12917–22
Min MY, Melyan Z, Kullmann DM. Synaptically released glutamate reduces gamma-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc Natl Acad Sci U S A 1999; 96: 9932–37
Chittajallu R, Vignes M, Dev KK, et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 1996; 379: 78–81
Cunha RA, Constantino MD, Ribeiro JA. Inhibition of [3H] γ-aminobutyric acid release by kainate receptor activation in rat hippocampal synaptosomes. Eur J Pharmacol 1997; 323: 167–72
Lerma J. Kainate receptors: an interplay between excitatory and inhibitory synapses. FEBS Lett 1998; 430: 100–4
Malva JO, Carvalho AP, Carvalho CM. Kainate receptors in hippocampal CA3 subregion: evidence for a role in regulating neurotransmitter release. Neurochem Int 1998; 32: 1–6
Perkinton MS, Sihra TS. A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes). Neuroscience 1999; 90: 1281–92
Liu QS, Patrylo PR, Gao XB, et al. Kainate acts at presynaptic receptors to increase GABA release from hypothalamic neurons. J Neurophysiol 1999; 82: 1059–62
Bigge CF, Nikam SS. AMPA receptor agonists, antagonists and modulators: their potential for clinical utility. Exp Opin Ther Patents 1997; 7: 1099–114
Löscher W, Lehmann H, Behl B, et al. A new pyrrolyl-quinoxalinedione series of non-NMDA glutamate receptor antagonists: pharmacological characterization and comparison with NBQX and valproate in the kindling model of epilepsy. Eur J Neurosci 1999; 11: 250–62
O’Neill MJ, Bond A, Ornstein PL, et al. Decahydro-isoquinolines: novel competitive AMPA/kainate antagonists with neuroprotective effects in global cerebral ischaemia. Neuropharmacology 1998; 37: 1211–22
Pirotte, B, Podona, T, Diouf, O, et al. 4H-l,2,4-pyridothiadiazine 1,1-dioxides and 2,3-dihydro-4H-1,2,4-pyridothiadiazine 1,1-dioxides chemically related to diazoxide and cyclothiazide as powerful positive allosteric modulators of (R/S)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-y1)propionic acid receptors: design, synthesis, pharmacology, and structure-activity relationships. J Med Chem 1998; 41: 2946–59
Wahl P, Frandsen A, Madsen U, et al. Pharmacology and toxicology of ATOA, an AMPA receptor antagonist and a partial agonist at GluR5 receptors. Neuropharmacology 1998; 37: 1205–10
Yaghoubi N, Malayev A, Russek SJ, et al. Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Res 1998; 803: 153–60
Barnard EA, Skolnick P, Olsen RW, et al. International union of pharmacology: XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 1998; 50: 291–314
Wilding TJ, Huettner JE. Antagonist pharmacology of kainate- and α-amino-3 -hydroxy-5-methyl-4-isoxazolepropionic acid-preferring receptors. Mol Pharmacol 1996; 49: 540–6
Lubisch W, Behl B, Hofmann HP. Pyrrolylquinoxalinediones: the importance of pyrrolic substitution on AMPA receptor binding. Biorg Med Chem Lett 1997; 7: 1101–6
Mutel V, Trube G, Klingelschmidt A, et al. Binding characteristics of a potent AMPA receptor antagonist [3H]Ro 488587 in rat brain. J Neurochem 1998; 71: 418–26
Sheardown MJ, Nielsen EØ, Hansen AJ, et al. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 1990; 247: 571–4
Ohmori J, Shimizu-Sasamata M, Okada M, et al. 8-(lH-im-idazol-1-yl)-7-nitro-4(5H)-imidazo[1,2-alpha]quinoxalino ne and related compounds: synthesis and structure-activity relationships for the AMPA-type non-NMDA receptor. J Med Chem 1997; 40: 2053–63
Kohara A, Okada M, Tsutsumi R, et al. In vitro characterization of YM872, a selective, potent and highly water-soluble α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist. J Pharm Pharmacol 1998; 50: 795–801
Takahashi M, Ni JW, Kawasaki-Yatsugi S, et al. YM872, a novel selective α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist, reduces brain damage after permanent focal cerebral ischemia in cats. J Pharmacol Exp Ther 1998; 284: 467–73
Turski L, Huth A, Sheardown M, et al. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci U S A 1998; 95: 10960–5
Paternain AV, Vicente A, Nielsen EØ, et al. Comparative antagonism of kainate-activated kainate and AMPA receptors in hippocampal neurons. Eur J Neurosci 1996; 8: 2129–36
Auberson YP, Acklin P, Allgeier H, et al. 5-Aminomethyl-quinoxaline-2,3-diones — Part II - N-aryl derivatives as novel NMDA/glycine and AMPA antagonists. Bioorg Med Chem Lett 1998; 8: 71–4
Bleakman D, Schoepp DD, Ballyk B, et al. Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3 carboxylic-acid. Mol Pharmacol 1996; 49: 581–5
Cai SX. Huang JC, Espitia SA, et al. 5-(N-oxyaza)-7-substi-tuted-1,4-dihydroquinoxaline-2,3-diones: novel, systemically active and broad spectrum antagonists for NMDA/glycine, AMPA, and kainate receptors. J Med Chem 1997; 40: 3679–86
Desos P, Lepagnol JM, Morain P, et al. Structure-activity relationships in a series of 2(1H)-quinolones bearing different acidic function in the 3-position: 6,7-dichloro-2(lH)oxoquinoline-3-phosphonic acid, a new potent and selective AMPA/kainate antagonist with neuroprotective properties. J Med Chem 1996; 39: 197–206
Nijholt I, Blank T, Grafelmann B. NS-257, a novel competitive AMPA receptor antagonist, interacts with kainate and NMDA receptors. Brain Res 1999; 821: 374–82
Sun GP, Slavica M, Uretsky NJ, et al. Design and synthesis of enantiomers of 3,5-dinitro-o-tyrosine α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonists. J Med Chem 1998; 41: 1034–41
Thomas NK, Clayton P, Jane DE. Dicarboxyphenylglycines antagonize AMPA- but not kainate-induced depolarizations in neonatal rat motoneurones. Eur J Pharmacol 1997; 338: 111–6
Vignes M, Bleakman D, Lodge D, et al. The synaptic activation of the GluR5 subtype of kainate receptor in area CA3 of the rat hippocampus. Neuropharmacology 1997; 36: 1477–81
Wahl P, Anker C, Traynelis SF, et al. Antagonist properties of a phosphono isoxazole amino acid at glutamate R1-4 (R,S)- 2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid receptor subtypes. Mol Pharmacol 1998; 53: 590–6
Hennegriff M, Arai A, Kessler M, et al. Stable expression of recombinant AMPA receptor subunits — binding affinities and effects of allosteric modulators. J Neurochem 1997; 68: 2424–34
Jane DE, Hoo K, Kamboj R, et al. Synthesis of willardiine and 6-azawillardiine analogs: pharmacological characterization on cloned homomeric human AMPA and kainate receptor subtypes. J Med Chem 1997; 40: 3645–50
Blaschke, M, Gremmels D, Everts I, et al. Pharmacological differentiation between neuronal and recombinant glutamate receptor channels expressed in Xenopus oocytes. Neuropharmacology 1997; 36: 1489–501
Bleakman D, Ballyk BA, Schoepp DD, et al. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology 1996; 35: 1689–702
Johansen TH, Chaudhary A, Verdoorn TA. Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol Pharmacol 1995; 48: 946–55
Löscher W. Pharmacology of glutamate receptor antagonists in the kindling model of epilepsy. Prog Neurobiol 1998; 54: 721–41
Magazanik LG, Buldakova SL, Samoilova MV, et al. Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J Physiol (Lond) 1997; 505: 655–63
Sekiguchi M, Fleck MW, Mayer ML, et al. A novel allosteric potentiator of AMPA receptors: 4-[2-(phenylsulfonyl-amino)ethylthio]-2,6-difluoro-phenoxyacetamide. J Neurosci 1997; 17: 5760–71
Varney MA, Rao SP, Jachec C, et al. Pharmacological characterization of the human ionotropic glutamate receptor subtype GluR3 stably expressed in mammalian cells. J Pharmacol Exp Ther 1998; 285: 358–70
Curry K, Pajouhesh H. Pharmacological profile of the isomers of the GluR-specific agonist ATPA. Can J Physiol Pharmacol 1998; 76: 690–2
Hoo K, Legutko B, Rizkalla G, et al. [3H]-ATPA: a high affinity ligand for GluR5 kainate receptors. Neuropharmacol 1999; 38: 1811–7
Stensbol TB, Borre L, Johansen TN, et al. Resolution, absolute stereochemistry and molecular pharmacology of the enantiomers of ATPA. Eur J Pharmacol 1999; 380: 153–62
Skjaerbaek N, Brehm L, Johansen TN, et al. Aryl and cycloalkyl analogues of AMPA: synthetic, pharmacological and stereo-chemical aspects. Bioorg Med Chem 1998; 6: 119–31
Johansen TN, Ebert B, Braunerosborne H, et al. Excitatory amino acid receptor ligands: resolution, absolute stereochemistry, and enantiopharmacology of 2-amino-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid. J Med Chem 1998; 41: 930–9
Banke TG, Lambert JD. Novel potent AMPA analogues differentially affect desensitisation of AMPA receptors in cultured hippocampal neurons. Eur J Pharmacol 1999; 367: 405–12
Conti P, De Amici M, De Sarro G, et al. Synthesis and enantiopharmacology of new AMPA-kainate receptor agonists. J Med Chem 1999; 42: 4099–107
Donevan SD, Beg A, Gunther JM, et al. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther 1998; 285: 539–45
Ramilles G, Swandulla D, Collingridge GL, et al. Interactions of 2,3 benzodiazepines and cyclothiazide at AMPA receptors; patch clamp recordings in cultured neurons and area CA1 in hippocampal slices. BrJ Pharmacol 1996; 117: 1209–21
Yamada KA, Turetsky DM. Allosteric interactions between cyclothiazide and AMPA/kainate receptor antagonists. Br J Pharmacol 1996; 117: 1663–72
Vizi ES, Mike A, Tarnawa I. The functional study of kainate receptors: hopes and doubts. Trends Neurosci 1997; 20: 396
Chimirri A, De Sarro G, De Sarro A, et al. l-Aryl-3,5-dihydro-4H-2,3-benzodiazepin-4-ones: novel AMPA receptor antagonists. J Med Chem 1997; 40: 1258–69
Chimirri A, De Sarro G, De Sarro A, et al. 3,5-Dihydro-4H-2,3-benzodiazepine-4-thiones: a new class of AMPA receptor antagonists. J Med Chem 1998; 41: 3409–16
Pelletier JC, Hesson DP, Jones KA, et al. Substituted 1,2-dihydrophthalazines: potent, selective, and non-competitive inhibitors of the AMPA receptor. J Med Chem 1996; 39: 343–6
Wang Y, Konkoy CS, Ilyin VI, et al. Synthesis of 7,8-(methylenedioxy)-1-phenyl-3,5-dihydro-4H-2,3-benzodiaz epin-4-ones as novel and potent noncompetitive AMPA receptor antagonists. J Med Chem 1998; 41: 2621–25
Arai A, Silberg J, Kessler M, et al. Effect of thiocyanate on AMPA receptor-mediated responses in excised patches and hippocampal slices. Neuroscience 1995; 66: 815–27
Bowie D, Smart TG. Thiocyanate ions selectively antagonize AMPA-evoked responses in Xenopus laevis oocytes microinjected with rat brain mRNA. Br J Pharmacol 1993; 109: 779–87
Eugène D, Moss SJ, Smart TG. Thiocyanate ions inhibit AMPA-activated currents in recombinant non-NMDA receptors expressed in Xenopus laevis oocytes: the role of the GluR2 subunit. Eur J Neurosci 1996; 8: 1983–93
Arai A, Kessler M, Ambros-Ingerson J, et al. Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience 1996; 75: 573–85
Arai A, Kessler M, Rogers G, et al. Effects of a memory-enhancing drug on DL-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther 1996; 278: 627–38
Kessler M, Mutneja MS, Rogers G, et al. Regional preferences of AMPA receptor modulators determined through agonist binding autoradiography. Brain Res 1998; 783: 121–6
Sekiguchi M, Takeo J, Harada T, et al. Pharmacological detection of AMPA receptor heterogeneity by use of two allosteric potentiators in rat hippocampal cultures. Br J Pharmacol 1998; 123: 1294–303
Nakagawa T, Iino M, Sekiguchi M, et al. Potentiating effects of 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluorphenoxya cetamide (PEPA) on excitatory synaptic transmission in dentate granule cells. Neurosci Res 1999; 35: 217–23
Larson J, Le T-T, Hall RA, et al. Effects of cyclothiazide on synaptic responses in slices of adult and neonatal hippocampus. NeuroReport 1994; 5: 389–92
Bähring R, Mayer ML. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J Physiol (Lond) 1998; 509: 635–50
Bähring R, Bowie D, Benveniste M, et al. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Lond) 1997; 502: 575–89
lino M, Koike M, Isa T, et al. Voltage-dependent blockage of Ca2+-permeable AMPA receptors by Joro spider toxin in cultured rat hippocampal neurones. J Physiol 1996; 496: 431–7
Savidge JR, Bristow DR. Ca2+ permeability and Joro spider toxin sensitivity of AMPA and kainate receptors on cerebellar granule cells. Eur J Pharmacol 1998; 351: 131–8
Buldakova SL, Vorobjev VS, Sharonova IN, et al. Characterization of AMPA receptor populations in rat brain cells by the use of subunit-specific open channel blocking drug, IEM-1460. Brain Res 1999; 846: 52–8
Samoilova MV, Buldakova SL, Vorobjev VS, et al. The open channel blocking drug, IEM-1460, reveals functionally distinct alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors in rat brain neurons. Neuroscience 1999; 94: 261–8
Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci 1998; 18: 8175–85
Oguro K, Oguro N, Kojima T, et al. Knockdown of AMPA or GluR2 expression causes delayed neurodegeneration and increases damage by sublethal ischemia in hippocampal CA1 and CA3 neurons. J Neurosci 1999; 19: 9218–27
Nishimune A, Isaac JT, Molnar E, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron 1998; 21: 87–97
Song I, Kamboj S,XiaJ, et al. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron 1998; 21: 393–400
Li Y, Hu XT, Berney TG, et al. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse 1999; 34: 169–80
Lüthi A, Chittajallu R, Duprat F, et al. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 1999; 24: 389–99
Kaczmarek L, Kossut M, Skangiel-Kramska J. Glutamate receptors in cortical plasticity: molecular and cellular biology. Pharmacol Rev 1997; 77: 217–55
Breese CR, Logel J, Adams C, et al. Regional gene expression of the glutamate receptor subtypes GluR1, GluR2, and GluR3 in human postmortem brain. J Mol Neurosci 1996; 7: 277–89
Day NC, Williams TL, Ince PG, et al. Distribution of AMPA selective glutamate receptor subunits in the human hippocampus and cerebellum. Mol Brain Res 1995; 31: 17–32
Hof PR, Vissavajjhala P, Rosenthal RE, et al. Distribution of glutamate receptor subunit proteins GluR2(4), GluR5/6/7, and NMDAR 1 in the canine and primate cerebral cortex: a comparative immunohistochemical analysis. Brain Res 1996; 723: 77–89
Ikonomovic MD, Sheffield R, Armstrong DM. AMPA-selective glutamate receptor subtype immunoreactivity in the aged human hippocampal formation. J Comp Neurol 1995;359: 239–52
Ong WY, He Y, Tan KK, et al. Differential localisation of the metabotropic glutamate receptor mGluRla and the ionotropic glutamate receptor GluR2/3 in neurons of the human cerebral cortex. Exp Brain Res 1998; 119: 367–74
Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res 1997; 751: 217–31
Tomiyama M, Palacios JM, Cortes R, et al. Distribution of AMPA receptor subunit mRNAs in the human basal ganglia: an in situ hybridization study. Mol Brain Res 1997; 46: 281–9
Tomiyama M, Rodriguez-Puertas R, Cortes R, et al. Differential regional distribution of AMPA receptor subunit messenger RNAs in the human spinal cord as visualized by in situ hybridization. Neuroscience 1996; 75: 901–15
Tomiyama M, Palacios JM, Cortes R, et al. Flip and flop variants of AMPA receptor subunits in the human cerebellum: implication for the selective vulnerability of Purkinje cells. Synapse 1999; 31: 163–7
Vickers JC, Huntley GW, Hof PR, et al. Immunocytochemical localization of non-NMDA ionotropic excitatory amino acid receptor subunits in human neocortex. Brain Res 1995; 671: 175–80
Williams TL, Ince PG, Oakley AE, et al. An immunocytochemical study of the distribution of AMPA selective glutamate receptor subunits in the normal human motor system. Neuroscience 1996; 74: 185–98
Gill SS, Pulido OM, Mueller RW, et al. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res Bull 1998; 46: 429–34
Bertrand G, Gross R, Puech R, et al. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol 1992; 106: 354–9
McNair CJ, Baxter GJ, Kerr R, et al. Glutamate receptor subunits associated with rat sympathetic preganglionic neurons. Neurosci Lett 1998; 256: 29–32
Ruggiero DA; Gootman PM; Sica A. Presence of a non-NMDA receptor subtype in the sympathetic nervous system of neonatal swine. J Auton Nerv Syst 1998; 73: 101–8
Carlton SM, Chung K, Ding Z, et al. Glutamate receptors on postganglionic sympathetic axons. Neuroscience 1998; 83: 601–5
Coggeshall RE, Carlton SM. Evidence for an inflammation-induced change in the local glutamatergic regulation of postganglionic sympathetic efferents. Pain 1999; 83: 163–8
Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. Comp Neurol 1998; 391: 78–86
Kirchgessner AL, Liu MT, Alcantara F. Excitotoxicity in the enteric nervous system. J Neurosci 1997; 17: 8804–16
Liu MT, Rothstein JD, Gershon MD, et al. Glutamatergic enteric neurons. J Neurosci 1997; 17: 4764–84
Chen Q, Veenman L, Knopp K, et al. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience 1998; 83: 749–61
Lambolez B, Ropert N, Perrais D, et al. Correlation between kinetics and RNA splicing of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci U S A 1996; 93: 1797–802
Robinson D, Ellenberger H. Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol 1997; 389: 94–116
Spike RC, Kerr R, Maxwell DJ, et al. GluRl and GluR2/3 subunit of the AMPA-type glutamate receptor are associated with particular types of neurone in laminae I-III of the spinal dorsal horn of the rat. Eur J Neurosci 1998; 10: 324–33
Varoqueaux F, Leranth C. Neurochemical characterization of AMPA receptor-containing neurons in the mediolateral septal area of the rat. Exp Brain Res 1997; 114: 454–60
Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron 1997; 18: 939–50
Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1998; 1: 572–8
Yin HZ, Sensi SL, Carriedo SG, et al. Dendritic localization of Ca2+-permeable AMPA/kainate channelas in hippocampal neurons. J Comp Neurol 1999; 409: 250–60
Morari M, Sbrenna S, Marti M, et al. NMDA and non-NMDA ionotropic glutamate receptors modulate striatal acetylcholine release via preand postsynaptic mechanisms. J Neurochem 1998; 71: 2006–17
Patel DR, Croucher MJ. Evidence for a role of presynaptic AMPA receptors in the control of neuronal glutamate release in the rat forebrain. Eur J Pharmacol 1997; 332: 143–51
Charara A, Blankstein E, Smith Y. Presynaptic kainate receptors in the monkey striatum. Neuroscience 1999; 91: 1195–200
Martin LJ, Furuta A, Blackstone CD. AMPA receptor protein in developing rat brain: glutamate receptor-1 expression and localization change at regional, cellular, and subcellular levels with maturation. Neuroscience 1998; 83: 917–28
Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 1991; 6: 799–810
Steinhauser C, Gallo V. News on glutamate receptors in glial cells. Trends Neurosci 1996; 19: 339–45
Matute C, Sanchez-Góomez MV, Martinez-Millan L, et al. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A 1997; 94: 8830–5
Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res 1996; 46: 427–37
Garcia-Barcina JM, Matute C. AMPA-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Mol Brain Res 1998; 53: 270–6
Garcia-Barcina JM, Matute C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur J Neurosci 1996; 8: 2379–87
Diano S, Naftolin F, Horvath TL. Kainate glutamate receptors (GluR5-7) in the rat arcuate nucleus: relationship to tanycytes, astrocytes, neurons and gonadal steroid receptors. J Neuroendocrinol 1998; 10: 239–47
Fan D, Grooms SY, Araneda RC, et al. AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J Neurosci Res 1999; 57: 557–71
Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab 1997; 17: 290–300
Krizbai IA, Deli MA, Pestenacz A, et al. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res 1998; 54: 814–9
Dong HL, O’Brien RJ, Fung ET, et al. GRrP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 1997; 386: 279–84
O’Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol 1998; 8: 364–9
Wyszynski M, Kim E, Yang FC, et al. Biochemical and immunocytochemical characterization of GRIP, a putative AMPA receptor anchoring protein, in rat brain. Neuropharmacology 1998; 37: 1335–44
Srivastava S, Osten P, Vilim FS, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron 1998; 21: 581–91
Xia J, Zhang X, Staudinger J, et al. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK 1. Neuron 1999; 22: 179–87
Leonard AS, Davare MA, Hörne MC, et al. SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. JBiol Chem 1998; 273: 19518–24
Osten P, Srivastava S, Inman GJ, et al. The AMPA receptor GluR2 C terminus can mediate a reversible ATP-dependent interaction with NSF and α- and β-SNAPs. Neuron 1998; 21: 99–110
Burette A, Wyszynski M, Valtschanoff JG, et al. Characterization of the glutamate receptor interacting protein-immunopositive neurons in the cerebellum and cerebral cortex of the albino rat. J Comp Neurol 1999; 411: 601–12
Dev KK, Nishimune A, Henley JM, et al. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology 1999; 38: 635–44
Dong H, Zhang P, Song I, et al. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP 2. J Neurosci 1999; 19: 6930–41
Noel J, Ralph GS, Pickard L, et al. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 1999; 23: 365–76
Wyszynski M, Valtschanoff JG, Naisbitt S, et al. Association of AMPA receptors with a subset of glutamate receptor -interacting protein invivo. J Neurosci 1999; 19: 6528–37
Garcia EP, Mehta S, Blair LA, et al. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron 1998; 21: 727–39
Rubio ME, Wenthold RJ. Calnexin and the immunoglobulin binding protein (BiP) coimmunoprecipitate with AMPA receptors. J Neurochem 1999; 73: 942–8
Allison DW, Gelfand VI, Spector I, et al. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci 1998; 18: 2423–36
O’Brien RJ, Xu D, Petralia RS, et al. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 1999; 23: 309–23
Rao A, Kim E, Sheng M, et al. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci 1998; 18: 1217–29
Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci Lett 1997; 238: 41–4
Takumi Y, Ramirez-Leon V, Laake P, et al. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 1999; 2: 618–24
Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol 1999; 412: 292–302
Isaac JTR, Crair MC, Nicoll RA, et al. Silent synapses during development of thalamocortical inputs. Neuron 1997; 18: 269–80
Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 1996; 381: 71–5
Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol 1999; 41: 92–101
Li P, Kerchner GA, Sala C, et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nature Neurosci 1999; 2: 972–7
Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during paired-induced LTP in CA1 region of hippocampal slice. Nature 1995; 375: 400–4
Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 1995; 15: 427–34
Shi S-H, Hayashi Y, Petralia RS, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 1999; 284: 1811–6
Carroll RC, Lissin DV, von Zastrow M, et al. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nature Neurosci 1999; 2: 454–60
Lissin DV, Carroll RC, Nicoll RA, et al. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci 1999; 19: 1263–72
Carroll RC, Beattie EC, Xia H, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A 1999; 96: 14112–7
Luscher C, Xia H, Beattie EC, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 1999; 24: 649–58
Lissin DV, Gomperts SN, Carroll RC, et al. Activity differentially regulates the surface expression of synaptic AMPA and NMDA receptors. Proc Natl Acad Sci U S A 1998; 95: 7097–102
O’Brien RJ, Kamboj S, Ehlers MD, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 1998; 21: 1057–78
Mammen AL, Huganir RL, O’Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci 1997; 17: 7351–8
Horikawa HP, Nawa H. Turnover rates of the AMPA-type glutamate receptor GluR1 measured by transient gene expression. J Neurosci Methods 1998; 84: 173–9
Ben-Ari Y, Khazipov R, Leinekugel X, et al. GABAa, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci 1997; 20: 523–9
Rumpel S, Hatt H, Gottmann K. Silent synapses in the developing rat visual cortex: evidence for postsynaptic expression of synaptic plasticity. J Neurosci 1998; 18: 8863–74
Petralia RS, Esteban JA, Wang YX, et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci 1999; 2: 31–6
Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 1998; 393: 695–98
Danysz W, Zajaczkowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol 1995; 6: 455–474
Sang CN, Hostetter MP, Gracely RH, et al. AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology 1998; 89: 1060–7
Umemura K, Kondo K, Ikeda Y, et al. Pharmacokinetics and safety of the novel amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist YM90K in healthy men. J Clin Pharmacol 1997; 37: 719–27
Kobayashi T, Caringi D, Mokier DJ, et al. Effects of ventrolateral medullary AMPA-receptor antagonism on pressor response during muscle contraction. Am J Physiol 1997; 272: H2774–81
Sirvio J, Larson J, Quach CN, et al. Effects of pharmacologically facilitating glutamatergic transmission in the trisynaptic intrahippocampal circuit. Neuroscience 1996; 74: 1025–35
Yamada KA. AMPA receptor activation potentiated by the AMPA modulator 1 -BCP is toxic to cultured rat hippocampal neurons. Neurosci Lett 1998; 249: 119–22
Staubli U, Perez Y, Xu F, et al. Centrally active modulators of glutamate receptors facilitate the induction of long-term potentiation invivo. Proc Natl Acad Sci U S A 1994; 91: 11158–62
Hack NJ, Sluiter AA, Balázs R. AMPA receptors in cerebellar granule cells during development in culture. Dev Brain Res 1995; 87: 8755–61
Impagnatiello F, Oberto A, Longone P, et al. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide: a partial modulator of AMPA receptor desensitization devoid of neurotoxicity. Proc Natl Acad Sci U S A 1997; 94: 7053–8
Jensen JB, Schousboe A, Pickering DS. AMPA receptor-mediated excitotoxicity in neocortical neurons is developmentally regulated and dependent upon receptor desensitization. Neurochem Int 1998; 32: 505–13
John CA, Beart PM, Giardina SF, et al. Cyclothiazide and GYKI52466 modulate AMPA receptor-mediated apoptosis in cortical neuronal cultures. Neurosci Lett 1999; 268: 9–12
May PC, Robison PM. Cyclothiazide treatment unmasks AMPA excitotoxicity in rat primary hippocampal cultures. J Neurochem 1993; 60: 1171–4
Ohno K, Okada M, Tsutsumi R, et al. Characterization of cyclothiazide-enhanced kainate excitotoxicity in rat hippocampal cultures. Neurochem Int 1998; 32: 265–71
Yamada KA, Covey DF, Hsu CY, et al. The diazoxide derivative IDRA 21 enhances ischémie hippocampal neuron injury. Ann Neurol 1998; 43: 664–9
Beal MF. Role of excitotoxicity in human neurological disease. Curr Opin Neurobiol 1992; 2: 657–62
Bittigau P, Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol 1997; 12: 471–85
Brouillet E, Hantraye P, Ferrante RJ, et al. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A 1995; 92: 7105–9
Hirata A, Nakamura R, Kwak S, et al. AMPA receptor-mediated slow neuronal death in the rat spinal cord induced by long-term blockade of glutamate transporters with THA. Brain Res 1997; 771: 37–44
Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson’s disease. Mol Neurobiol 1996; 12: 73–94
Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 1998; 44 Suppl. 3: S175–88
Matute C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc Natl Acad Sci USA 1998; 95: 10229–34
Cebers G, Cebere A, Liljequist S. Metabolic inhibition potentiates AMPA-induced Ca2+ fluxes and neurotoxicity in rat cerebellar granule cells. Brain Res 1998; 779: 194–204
Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol 1996; 48: 613–34
Massieu L, Garcia O. The role of excitotoxicity and metabolic failure in the pathogenesis of neurological disorders. Neurobiology 1998; 6: 99–108
Parker WD, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 1990; 40: 1302–3
Chandrasekaran K, Giordano T, Brady DR, et al. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Mol Brain Res 1994; 24: 336–40
Chandrasekaran K, Hatanpaa K, Brady DR, et al. Downregulation of oxidative phosphorylation in Alzheimer disease: loss of cytochrome oxidase subunit mRNA in the hippocampus and entorhinal cortex. Brain Res 1998; 796: 13–9
Davis RE, Miller S, Herrnstadt C, et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1997; 94: 4526–31
Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 1994; 63: 2179–84
Parker WD, Parks J, Filley CM, et al. Electron transport chain defects in Alzheimer’s disease brain. Neurology 1994; 44: 1090–6
Sheehan JP, Swerdlow RH, Miller SW, et al. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci 1997; 17: 4612–22
Wong-Riley M, Antuono P, Ho KC, et al. Cytochrome oxidase in Alzheimer’s disease: biochemical, histochemical, and immunohistochemical analyses of the visual and other systems. Vision Res 1997; 37: 3593–608
Simonian NA, Hyman BT. Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropathol Exp Neurol 1994; 53: 508–12
Mecocci P, Beal MF, Cecchetti R, et al. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol Chem Neuropathol 1997; 31: 53–64
Redjems-Bennani N, Jeandel C, Lefebvre E, et al. Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology 1998; 44: 300–4
Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta 1998; 1366: 211–23
Sims NR. Energy metabolism, oxidative stress and neuronal degeneration in Alzheimer’s disease. Neurodegeneration 1996; 5: 435–40
Rapoport SI, Hatanpaa K, Brady DR, et al. Brain energy metabolism, cognitive function and down-regulated oxidative phosphorylation in Alzheimer disease. Neurodegeneration 1996; 5: 473–6
Schinder AF, Olson EC, Spitzer NC, et al. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 1996, 16: 6125–33
Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995; 15: 961–73
Nicholls DG, Budd SL. Mitochondria and neuronal glutamate excitotoxicity. Biochim Biophysica Acta 1998; 1366: 97–112
Montai M. Mitochondria, glutamate neurotoxicity and the death cascade. Biochim Biophys Acta 1998; 1366: 113–26
Nicotera P, Ankarcrona M, Bonfoco E, et al. Neuronal necrosis and apoptosis: two distinct events induced by exposure to glutamate or oxidative stress. Adv Neurol 1997; 72: 95–101
Cassarino DS, Bennett JP. An evaluation of the role of mitochondria in neurodegenerative diseases: mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Res Rev 1999; 29: 1–25
Nicotera P, Leist M, Manzo L. Neuronal cell death: a demise with different shapes. Trends Pharmacol Sci 1999; 20: 46–51
David JC, Yamada KA, Bagwe MR, et al. AMPA receptor activation is rapidly toxic to cortical astrocytes when desensitization is blocked. J Neurosci 1996; 16: 200–9
McDonald JW, Althomsons SP, Hyrc KL, et al. Oligoden-drocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. NatMed 1998; 4: 291–7
McDonald JW, Levine JM, Qu Y. Multiple classes of the oligodendrocyte lineage are highly vulnerable to excitotoxicity. NeuroReport 1998; 9: 2757–62
Sánchez-Gómez MV, Matute C. AMPA and kainate receptors each mediate excitotoxicity in oligodendroglial cultures. Neurobiol Dis 1999; 6: 475–85
Solum D, Hughes D, Major MS, et al. Prevention of normally occurring and deafferentation-induced neuronal death in chick brainstem auditory neurons by periodic blockade of AMPA/kainate receptors. J Neurosci 1997; 17: 4744–51
Tandon P, Liu Z, Stafstrom CE, et al. Long-term effects of excitatory amino acid antagonists NBQX and MK801 on the developing brain. Dev Brain Res 1996; 95: 256–62
Lees GJ. Therapeutic potential of AMPA receptor ligands in neurological disorders. CNS Drugs 1996; 5: 51–74
Ingwersen SH, Ohrstrom JK, Petersen P, et al. Human pharmacokinetics of the neuroprotective agent NBQX. Am J Therap 1994; 1: 296–303
Rizzo M, De Sarro G, Zappala M, et al. Determination of new 2,3-benzodiazepines in rat plasma using high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl 1999; 731: 207–15
Xue D, Huang ZG, Barnes K, et al. Delayed treatment with AMPA, but not NMDA, antagonists reduces neocortical infarction. J Cereb Blood Flow Metab 1994; 14: 251–61
Yatsugi S, Takahashi M, Kawasaki-Yatsugi S, et al. Neuroprotective effect of YM9OK, a novel AMPA/kainate receptor antagonist, in focal cerebral ischemia in cats. J Cereb Blood Flow Metab 1996; 16: 959–66
Hampson RE, Rogers G, Lynch G, et al. Facilitative effects of the ampakine CX516 on short-term memory in rats — correlations with hippocampal neuronal activity. J Neurosci 1998; 18: 2748–63
Lynch G, Kessler M, Rogers G, et al. Psychological effects of a drug that facilitates brain AMPA receptors. Int Clin Psychopharmacol 1996; 11: 13–9
Hampson RE, Rogers G, Lynch G, et al. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci 1998; 18: 2740–7
Taubøll E, Gjerstad L. Effects of antiepileptic drugs on the activation of glutamate receptors. Prog Brain Res 1998; 116: 385–47
Ingvar M, Ambros-Ingerson J, Davis M, et al. Enhancement by an ampakine of memory encoding in humans. Exp Neurol 1997; 146: 553–9
Lynch G, Granger R, Ambros-Ingerson J, et al. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol 1997; 145: 89–92
Riedell G, Micheaul, J, Lam AGM, et al. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature Neurosci 1999; 2: 898–905
Filliat P, Pernotmarino I, Baubichon D, et al. Behavioral effects of NBQX, a competitive antagonist of AMPA receptors. Pharmacol Biochem Behav 1998; 59: 1087–92
Parada J, Czuczwar SJ, Turski WA. NBQX does not affect learning and memory tasks in mice: a comparison with DCPPene and ifenprodil. Cogn Brain Res 1992; 1: 67–71
Li HB, Matsumoto K, Yamamoto M, et al. NMDA but not AMPA receptor antagonists impair the delay-interposed radial maze performance of rats. Pharmacol Biochem Behav 1998; 58: 249–53
Stephens DN, Cole BJ. AMPA antagonists differ from NMDA antagonists in their effects on operant DRL and delayed matching to position tasks. Psychopharmacology 1996; 126: 249–59
Dürmüller N, Craggs M, Meldrum BS. The effect of the non-NMDA receptor antagonists GYKI 52466 and NBQX and the competitive NMDA receptor antagonist D-CPPene on the development of amygdala kindling and on amygdala-kindled seizures. Epilepsy Res 1994; 17: 167–74
Danysz W, Essmann U, Bresink I, et al. Glutamate antagonists have different effects on spontaneous locomotor activity in rats. Pharmacol Biochem Behav 1994; 48: 111–8
Maj J, Rogoz Z, Skuza G, et al. Some behavioral effects of CNQX and NBQX, AMPA receptor antagonists. Pol J Pharmacol 1995; 47: 269–77
Maj J, Rogoz Z, Skuza G, et al. Some central effects of GYKI 52466, a non-competitive AMPA receptor antagonist. Pol J Pharmacol 1995; 47: 501–7
Larson J, Lieu T, Petchpradub V, et al. Facilitation of olfactory learning by a modulator of AMPA receptors. J Neurosci 1995; 15: 8023–30
Davis CM, Moskovitz B, Nguyen MA, et al. A profile of the behavioral changes produced by facilitation of AMPA-type glutamate receptors. Psychopharmacol 1997; 133: 161–7
Kubova H, Vilagi I, Mikulecka A, et al. Non-NMDA receptor antagonist GYKI 52466 suppresses cortical afterdischarges in immature rats. Eur J Pharmacol 1997; 333: 17–26
Nishiyama T, Yaksh TL, Weber E. Effects of intrathecal NMDA and non-NMDA antagonists on acute thermal nociception and their interaction with morphine. Anesthesiology 1998; 89: 715–22
De Sarro G, Di Paola ED, Gareri P, et al. Effects of some AMPA receptor antagonists on the development of tolerance in epilepsy-prone rats and in pentylenetetrazole kindled rats. Eur J Pharmacol 1999; 368: 149–59
Furuya N, Koizimi T, Sebata H. Effects of newly developed excitatory amino acid antagonists on vestibular type I neurons in the cat. Acta Oto-Laryngologica Suppl (Stockh) 1997; 528: 52–5
Manfridi A, Brambilla D, Mancia M. Stimulation of NMDA and AMPA receptors in the rat nucleus basalis of Meynert affects sleep. Am J Physiol 1999; 277: R1488–92
Onoe H, Saki K. Kainate receptors: a novel mechanism in paradoxical (REM) sleep generation. Neuroreport 1995; 6: 353–6
Zhang XF, Hu XT, White FJ, et al. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther 1997; 281: 699–706
Ghasemzadeh MB, Nelson LC, Lu XY, et al. Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J Neurochem 1999; 72: 157–65
Carlezon WA, Rasmussen K, Nestler EJ. AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse 1999; 31: 256–62
Jackson A, Mead AN, Rocha BA, et al. AMPA receptors and motivation for drug: effect of the selective antagonist NBQX on behavioural sensitization and on self-administration in mice. Behav Pharmacol 1998; 9: 457–67
Li Y, Vartanian AJ, White FJ, et al. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology 1997; 134: 266–76
Mead AN, Stephens DN. AMPA-receptors are involved in the expression of amphetamine-induced behavioural sensitisation, but not in the expression of amphetamine-induced conditioned activity in mice. Neuropharmacology 1998; 37: 1131–8
Mead AN, Vasilaki A, Spyraki C, et al. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. EurJNeurosci 1999; 11: 4089–98
Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Proc Neurobiol 1998; 54: 679–720
Akiyama K, Ujike H, Sakai K, et al. Effect of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline on methamphet-amine- and cocaine-induced behavioral sensitization. Pharmacol Biochem Behav 1998; 61: 419–26
Carlezon WA, Boundy VA, Haile CN, et al. Sensitization to morphine induced by viral-mediated gene transfer. Science 1997; 277: 812–4
Granger R, Staubli U, Davis M, et al. A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse 1993; 15: 326–9
Larson J, Quach CN, LeDuc B, et al. Effects of an AMPA receptor modulator on methamphetamine-induced hyperactivity in rats. Brain Res 1996; 738: 353–6
Palmer LC, Hess US, Larson J, et al. Comparison of the effects of an ampakine with those of methamphetamine on aggregate neuronal activity in cortex versus striatum. Mol Brain Res 1997; 46: 127–35
Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J Physiol (Lond) 1998; 509: 255–66
Bongianni F, Mutolo D, Carfi M, et al. Area postrema glutamate receptors mediate respiratory and gastric responses in the rabbit. NeuroReport 1998; 9: 2057–62
Bissonnette JM, Hohimer AR, Knopp SJ. Non-NMDA receptors modulate respiratory drive in fetal sheep. J Physiol (Lond) 1997; 501: 415–23
Haxhiu MA, Erokwu B, Dreshaj IA. The role of excitatory amino acids in airway reflex responses in anesthetized dogs. J Auton Nerv Syst 1997; 67: 192–9
Shimazu Y, Umemura K, Kawano K-I, et al. Respiratory effects of halothane and AMPA receptor antagonist synergy in rats. Eur J Pharmacol 1998; 342: 261–5
Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. J Pharmacol Exp Ther 1997; 282: 639–47
Lillaney R, Maher TJ, Chaiyakul P, et al. Changes in extracellular glutamate and pressor response during muscle contraction following AMPA-receptor blockade in the RVLM and CVLM. Brain Res 1999; 844: 164–73
Miyawaki T, Suzuki S, Minson J, et al. Role of AMPA/kainate receptors in transmission of the sympathetic baroreflex in rat CVLM. Am J Physiol 1997; 272: R800–12
Teng YD, Wrathall JR. Evaluation of cardiorespiratory parameters in rats after spinal cord trauma and treatment with NBQX, an antagonist of excitatory amino acid receptors. Neurosci Lett 1996; 209: 5–8
Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res 1997; 89: 107–13
Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res 1998; 93: 43–50
Xu Z, Johnson AK. Non-NMDA receptor antagonist-induced drinking in rat. Brain Res 1998; 808: 124–7
Ping L, Mahesh VB, Bhat GK, et al. Regulation of gonadotropin-releasing hormone and luteinizing hormone secretion by AMPA receptors. Evidence for a physiological role of AMPA receptors in the steroid-induced luteinizing hormone surge. Neuroendocrinology 1997; 66: 246–53
Zuo Z, Mahesh VB, Zamoizano PL et al. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology 1996; 137: 2334–38
Brann DW, Mahesh VB. Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. EndocrRev 1997; 18: 678–700
Mahesh VB, Zamorano P, De Sevilla L, et al. Characterization of ionotropic glutamate receptors in rat hypothalamus, pituitary and immortalized gonadotrophin-releasing hormone (GnRH) neurons (GT1-7 cells). Neuroendocrinol 1999; 69: 397–407
Sladek CD, Badre SE, Morsette DJ, et al. Role of non-NMDA receptors and glutamate stimulation of vasopressin release: effect of rapid receptor desensitization. J Neuroendocrinol 1998; 10: 897–903
Gonzalez LC, Pinilla L, Tena-Sempere M, et al. Regulation of growth hormone secretion by a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in infantile, prepubertal, and adult male rats. Endocrinology 1999; 140: 1279–84
Tokarev D, Jezova D. Effect of central administration of the non-NMDA receptor antagonist DNQX on ACTH and corticosterone release before and during immobilization stress. Meth Find Exp Clin Pharmacol 1997; 19: 323–8
Gonzalez LC, Pinilla L, Tena-Sempere M, et al. Role of alphaamino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in the control of prolactin, growth hormone and gonadotropin secretion in prepubertal rats. J Endocrinol 1999; 162: 417–24
Weaver CD, Partridge JG, Yao TL, et al. Activation of glycine and glutamate receptors increases intracellular calcium in cells derived from the endocrine pancreas. Mol Pharmacol 1998; 54: 639–46
Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther 1997; 280: 894–904
Nishizawa O, Igawa Y, Satoh T, et al. Effects of glutamate receptor antagonists on lower urinary tract function in conscious unanesthetized rats. Adv Exp Biol Med 1999; 462: 275–81
Gill R, Lodge D. Pharmacology of AMPA antagonists and their role in neuroprotection. Int Rev Neurobiol 1997; 40: 197–232
Ikonomidou C, Turski L. Pharmacology of the AMPA antagonist 2,3 -dihydroxy-6-nitro-7-sulfamoylbenzo-(F)-quinoxaline. Ann N Y Acad Sci 1997; 825: 394–402
Rogers DC, Hunter AJ. Dissociation of effects of glutamate receptor antagonists on excitotoxic and hypoxic neuronal cell death in a novel rat cortical culture system. Brain Res Bull 1997; 44: 131–9
De Keyser J, Suiter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci 1999; 22: 535–40
Lazarewicz JW, Gadamski R, Parsons CG, et al. Protection against post-ischaemic neuronal loss in gerbil hippocampal CA1 by glycineB and AMPA antagonists. J Neural Transm 1997; 104: 1249–54
Nurse S, Corbett D. Neuroprotection after several days of mild, drug-induced hypothermia. J Cereb Blood Flow Metab 1996; 16: 474–80
Busto R, Dietrich WD, Globus MY, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987; 7: 729–38
Xue D, Huang ZG, Smith KE, et al. Immediate or delayed mild hypothermia prevents focal cerebral infarction. Brain Res 1992; 587: 66–72
Meng SZ, Ohyu J, Takashima S. Changes in AMPA glutamate and dopamine D2 receptors in hypoxic-ischemic basal ganglia necrosis. Pediatr Neurol 1997; 17: 139–43
Ben-Ari Y, Khrestchatisky M. The GluR2 (GluRB) hypothesis in ischemia: missing links. Trends Neurosci 1998; 21: 241–2
Prince HK, Conn PJ, Blackstone CD, et al. Down-regulation of AMPA receptor subunit GluR2 in amygdaloid kindling. J Neurochem 1995; 64: 462–5
Pollard H, Héron A, Moreau J, et al. Alterations of the GluR-B AMPA receptor subunit flip/flop expression in kainate-in-duced epilepsy and ischemia. Neuroscience 1993; 57: 545–54
Friedman LK, Pellegrini-Giampietro DE, Sperber EF, et al. Kainate-induced status epilepticus alters glutamate and GABAa receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci 1994; 14: 2697–707
Friedman LK. Selective reduction of GluR2 protein in adult hippocampal CA3 neurons following status epilepticus but prior to cell loss. Hippocampus 1998; 8: 511–25
Standley S, Baudry M. Rapid effects of kainate administration on α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor properties in rat hippocampus. Exp Neurol 1998; 152: 208–13
Friedman LK, Sperber EF, Moshé SL, et al. Developmental regulation of glutamate and GABAa receptor gene expression in rat hippocampus following kainate-induced status epilepticus. Dev Neurosci 1997; 19: 529–42
Friedman LK, Velisková J. GluR2 hippocampal knockdown reveals developmental regulation of epileptogenicity and neurodegeneration. Mol Brain Res 1998; 61: 224–31
Friedman LK, Koudinov AR. Unilateral GluR2(B) hippocampal knockdown: a novel partial seizure model in the developing rat. J Neurosci 1999; 19: 9412–25
Lason W, Turchan J, Przewlocka B, et al. Effects of pentylenetetrazol kindling on glutamate receptor genes expression in the rat hippocampus. Brain Res 1998; 785: 355–8
Lason W, Turchan J, Przewlocka B, et al. Scizure-related changes in the glutamate R2 and R5 receptor genes expression in the rat hippocampal formation. J Neural Transm 1997; 104: 125–33
Blumcke I, Beck H, Scheffler B, et al. Altered distribution of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit GluR2(4) and the N-methyl-D-aspartate receptor subunit NMDAR1 in the hippocampus of patients with temporal lobe epilepsy. Acta Neuropathol (Berl) 1996; 92: 576–87
Brines ML, Sundaresan S, Spencer DD, et al. Quantitative autoradiographic analysis of ionotropic glutamate receptor sub-types in human temporal lobe epilepsy: up-regulation in reorganized epileptogenic hippocampus. Eur J Neurosci 1997; 9: 2035–44
Grigorenko EV, Bell WL, Glazier S, et al. Editing status at the Q/R site of the GluR2 and GluR6 glutamate receptor subunits in the surgically excised hippocampus of patients with refractory epilepsy. NeuroReport 1998; 9: 2219–24
Grigorenko E, Glazier S, Bell W, et al. Changes in glutamate receptor subunit composition in hippocampus and cortex in patients with refractory epilepsy. J Neurol Sci 1997; 153: 35–45
Mathern GW, Pretorius JK, Kornblum HI, et al. Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol Dis 1998; 5: 151–76
Mathern GW, Pretorius JK, Kornblum HI, et al. Human hippocampal AMPA and NMDA mRNA levels in temporal lobe epilepsy patients. Brain 1997; 120: 1937–59
Ying Z, Babb TL, Comair YG, et al. Induced expression of NMDAR2 proteins and differential expression of NMDAR1 splice variants in dysplastic neurons of human epileptic neocortex. J Neuropathol Exp Neurol 1998; 57: 47–62
Zilles K, Qu MS, Kohling R, et al. Ionotropic glutamate and GABA receptors in human epileptic neocortical tissue: quantitiative in vitro receptor autoradiography. Neuroscience 1999; 94: 1051–61
Carlton SM, Hargett GL, Coggeshall RE. Plasticity in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor sub-units in the rat dorsal horn following deafferentation. Neurosci Lett 1998; 242: 21–4
Lason W, Turchan J, Przewlocka B, et al. Effects of repeated MK-801 administration on the glutamate receptor gene expression in the rat hippocampus. Pol J Pharmacol 1997; 49: 249–53
Nair SM, Werkman TR, Craig J, et al. Corticosteroid regulation of ion channel conductances and m-RNA levels in individual hippocampal CA1 neurons. J Neurosci 1998; 18: 2685–96
Boris-Moller F, Wieloch T. Changes in the extracellular levels of glutamate and aspartate during ischemia and hypoglycemia: effects of hypothermia. Exp Brain Res 1998; 121: 277–84
Caragine LP, Park HK, Diaz FG, et al. Real-time measurement of ischemia-evoked glutamate release in the cerebral cortex of four and eleven vessel rat occlusion models. Brain Res 1998; 793: 255–64
Meldrum BS. Excitotoxicity and selective neuronal loss in epilepsy. Brain Pathol 1993; 3: 405–12
Meldrum BS, Evans MC, Swan JH, et al. Protection against hypoxic/ischaemic brain damage with excitatory amino acid antagonists. Med Biol 1987; 65: 153–7
Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol 1997; 51: 39–87
Graham SH, Chen J, Lan JQ, et al. A dose-response study of neuroprotection using the AMPA antagonist NBQX in rat focal cerebral ischemia. J Pharmacol Exp Ther 1996; 276: 1–4
Kawasaki-Yatsugi S, Shimizu-Sasamata M, Yatsugi S, et al. Delayed treatment with YM9OK, an AMPA receptor antagonist, protects against ischaemic damage after middle cerebral artery occlusion in rats. J Pharm Pharmacol 1998; 50: 891–8
Lo EH, Pierce AR, Mandeville JB, et al. Neuroprotection with NBQX in rat focal cerebral ischemia: effects on ADC probability distribution functions and diffusion-perfusion relationships. Stroke 1997; 28: 439–46
Shimizu-Sasamata M, Kawasaki-Yatsugi S, Okada M, et al. YM9OK: pharmacological characterization as a selective and potent α-amino-3-hydroxy-5-methylisoxazole-4-propionate/ kainate receptor antagonist. J Pharmacol Exp Ther 1996; 276: 84–92
Umemura K, Shimakura A, Nakashima M. Neuroprotective effect of a novel AMPA receptor antagonist, YM9OK, in rat focal cerebral ischaemia. Brain Res 1997; 773: 61–5
Yao H, Ibayashi S, Nakane H, et al. AMPA receptor antagonist, YM9OK, reduces infarct volume in thrombotic distal middle cerebral artery occlusion in spontaneously hypertensive rats. Brain Res 1997; 753: 80–5
Kawasaki-Yatsugi S, Yatsugi S, Takahashi M, et al. A novel AMPA receptor antagonist, YM872, reduces infarct size after middle cerebral artery occlusion in rats. Brain Res 1998; 793: 39–46
Shimizu-Sasamata M, Kano T, Rogowska J, et al. YM872, a highly water-soluble AMPA receptor antagonist, preserves the hemodynamic penumbra and reduces brain injury after permanent focal ischemia in rats. Stroke 1998; 29: 2141–8
Schielke GP, Kupina NC, Boxer PA, et al. The neuroprotective effect of the novel AMPA receptor antagonist PD152247 (PNQX) in temporary focal ischemia in the rat. Stroke 1999; 30: 1472–7
Ni JW, Takahashi M, Yagsugi S, et al. Effects of YM872 on atrophy of substantia nigra reticulata after focal ischemia in rats. NeuroReport 1998; 9: 3179–24
Hu P, Diemer NH, Bruhn T, et al. Effects of the AMPA-receptor antagonist, NBQX, on neuron loss in dentate hilus of the hippocampal formation after 8, 10, or 12 min of cerebral ischemia in the rat. J Cereb Blood Flow Metab 1997; 17: 147–52
Kawasaki-Yatsugi S, Yatsugi S, Koshiya K, et al. Neuroprotective effect of YM9OK, an AMPA-receptor antagonist, against delayed neuronal death induced by transient global cerebral ischemia in gerbils and rats. Jpn J Pharmacol 1997; 74: 253–60
Hagberg H, Gilland E, Diemer NH, et al. Hypoxia-ischemia in the neonatal rat brain: histopathology after post-treatment with NMDA and non-NMDA receptor antagonists. Biol Neonate 1994; 66: 205–13
Brambrink AM, Martin LJ, Hanley DF et al. Effects of the AMPA receptor antagonist NBQX on outcome of newborn pigs after asphyxic cardiac arrest. J Cereb Blood Flow Metab 1999; 19: 927–38
Copin JC, Li Y, Reola L, et al. Trolox and 6,7-dinitroquinoxaline-2,3-dione prevent necrosis but not apoptosis in cultured neurons subjected to oxygen deprivation. Brain Res 1998; 784: 25–36
Katsumori H, Minabe Y, Osawa M, et al. Acute effects of various GABA receptor agonists and glutamate antagonists on focal hippocampal seizures in freely moving rats elicited by low-frequency stimulation. Synapse 1998; 28: 103–9
Tortorella A, Halonen T, Sahibzada N, et al. A crucial role of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype of glutamate receptors in piriform and perirhinal cortex for the initiation and propagation of limbic motor seizures. J Pharmacol Exp Therap 1997; 280: 1401–5
Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol 1999; 79: 947–63
Holmes GL. Do seizures cause brain damage? Epilepsia 1991; 32 Suppl. 5: S14–28
Wasterlain CG, Fujikawa DG, Penix L, et al. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 1993; 34 Suppl. 1: S37–53
Gillardon F, Bottiger B, Schmitz B, et al. Activation of CPP-32 protease in hippocampal neurons following ischemia and epilepsy. Mol Brain Res 1997; 50: 16–22
Lees GJ, Leong W. Synergy between diazepam and NBQX in preventing neuronal death caused by non-NMDA agonists. NeuroReport 1994; 5: 2149–52
Lees GJ, Leong W. Differential effects of NBQX on the distal and local toxicity of glutamate agonists administered intrahippocampally. Brain Res 1993; 628: 1–7
Andrews PI, McNamara JO. Rasmussen’s encephalitis: an autoimmune disorder? Curr Opin Neurobiol 1996; 6: 673–8
Andrews PI, McNamara JO, Lewis DV. Clinical and electroen-cephalographic correlates in Rasmussen’s encephalitis. Epilepsia 1997; 38: 189–94
McNamara JO, Patel M, He XP, et al. Glutamate receptor autoimmunity in Rasmussen’s encephalitis. Cold Spring Harb Symp Quant Biol 1996; 61: 327–32
Rogers SW, Andrews PI, Gahring LC, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science 1994; 265: 648–51
Krauss GL, Campbell ML, Roche KW, et al. Chronic steroid-responsive encephalitis without autoantibodies to glutamate receptor GluR 3. Neurology 1996; 46: 247–9
He XP, Patel M, Whitney KD, et al. Glutamate receptor GluR3 antibodies and death of cortical cells. Neuron 1998; 20: 153–63
Gahring LC, Carlson NG, Rogers SW. Antibodies prepared to neuronal glutamate receptor subunit3 bind IFNalpha-receptors: implications for an autoimmune process. Autoimmunity 1998; 28: 243–8
Levite M, Hermelin A. Autoimmunity to the glutamate receptor in mice: a model for Rasmussen’ s encephalitis? J Autoimmun 1999; 13: 73–82
Dambinova SA, Izykenova GA, Burov SV, et al. The presence of autoantibodies to N-terminus domain of GluR1 subunit of AMPA receptor in the blood serum of patients with epilepsy. J Neurol Sci 1997; 152: 93–7
Sander T, Hildmann T, Kretz R, et al. Allelic association of juvenile absence epilepsy with a GluR5 kainate receptor gene (GRIK1) polymorphism. Am J Med Genet 1997; 74: 416–21
Perucca E. A pharmacological and clinical review on topiramate, anew antiepileptic drug. Pharmacol Res 1997; 35: 241–56
Rosenfeld WE. Topiramate, a review of preclinical, pharmacokinetic and clinical data. Clin Ther 1997; 19: 1294–308
Piña-Garza JE, McLean MJ. Different effects of topiramate and phenytoin on mouse seizures and responses of cultured neurons to excitatory amino acids. Epilepsia 1996; 37 Suppl. 5: S26
Kunig G, Niedermeyer B, Deckert J, et al. Inhibition of [3H]α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid [AMPA] binding by the anticonvulsant valproate in clinically relevant concentrations: an autoradiographic investigation in human hippocampus. Epilepsy Res 1998; 31: 153–7
Kodama M, Yamada N, Sato K, et al. Effects of YM90K, a selective AMPA receptor antagonist, on amygdala-kindling and long-term potentiation in the rat. Eur J Pharmacol 1999; 374: 11–9
Potschka H, Löscher W, Wlaz P, et al. LU 73068, a new non-NMDA and glycine/NMDA receptor antagonist: pharmacological characterization and comparison with NBQX and L-701,324 in the kindling model of epilepsy. Br J Pharmacol 1998; 125: 1258–66
Foutz AS, Pierrefiche O, Denavit-Saubie M. Combined blockade of NMDA and non-NMDA receptors produces respiratory arrest in the adult cat. NeuroReport 1994; 5: 481–4
McManigle JE, Taveira DaSilva AM, Dretchen KL, et al. Potentiation of MK-801-induced breathing impairment by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline. Eur J Pharmacol 1994; 252: 11–7
Bigge CF, Malone TC, Boxer PA, et al. Synthesis of 1,4,7,8,9,10-hexahydro-9-methyl-6-nitropyrido[3,4-f]quin oxaline-2,3-dione and related quinoxalinediones: characterization of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (and N-methyl-D-aspartate) receptor and anticonvulsant activity. J Med Chem 1995; 38: 3720–40
Zarnowski T, Kleinrok Z, Turski WA, et al. 2,3-Dihydroxy-6-nitro-7-sulfainoylbenzo(f)quinoxaline enhances the protective activity of common antiepileptic drugs against maximal electroshock-induced seizures in mice. Neuropharmacology 1993; 32: 895–900
Borowicz KK, Gasior M, Kleinrok Z, et al. The non-competitive AMPA/kainate receptor antagonist, GYKI52466, potentiates the anticonvulsant activity of conventional antiepileptics. Eur J Pharmacol 1995; 281: 319–26
Czuczwar SJ, Swiader M, Kuzniar H. LY 300164, a novel antagonist of AMPA/kainate receptors, potentiates the anticonvulsive activity of antiepileptic drugs. Eur J Pharmacol 1998; 359: 103–9
Bullock R, Zauner A, Woodward JJ, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg 1998; 89: 507–18
Obrenovitch TP, Urenjak J. Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J Neurotrauma 1997; 14: 677–98
Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci 1994; 14: 6598–607
Wrathall JR, Teng YD, Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol 1996; 137: 119–26
Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol 1997; 145: 565–73
Follesa P, Wrathall JR, Mocchetti I. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)-quinoxaline (NBQX) increases fibroblast growth factor mRNA levels after contusive spinal cord injury. Brain Res 1998; 782: 306–9
Catts SV, Ward PB, Lloyd A, et al. Molecular biological investigations into the role of the NMDA receptor in the pathophysiology of schizophrenia. Aust NZ J Psychiat 1997; 31: 17–26
Deakin JFW, Simpson MDC. A two-process theory of schizophrenia — evidence from studies in post-mortem brain. J Psychiat Res 1997; 31: 277–95
Goff DC, Wine L. Glutamate in schizophrenia — clinical and research implications. Schizophrenia Res 1997; 27: 157–68
Hirsch SR, Das I, Garey LJ, et al. A pivotal role for glutamate in the pathogenesis of schizophrenia, and its cognitive dysfunction. Pharmacol Biochem Behav 1997; 56: 797–802
Ishimaru MJ, Tora M. The glutamate hypothesis of schizophrenia. Therapeutic Implications. CNS Drugs 1997; 7: 46–67
Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 1998; 12: 21–36
Tamminga C. Glutamatergic aspects of schizophrenia. Br J Psychiatry 1999; 174 Suppl. 37: 12–5
Carlsson A, Hansson LO, Waters N, et al. A glutamatergic deficiency model of schizophrenia. Br J Psychiatry 1999; 174 Suppl. 37: 2–6
Noga JT, Hyde TM, Herman MM, et al. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse 1997; 27: 168–76
Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res 1995; 674: 82–90
Eastwood SL, Burnet PW, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Mol Brain Res 1997; 44: 92–8
Eastwood SL, Kerwin RW, Harrison PJ. Immunoauto-radiographic evidence for a loss of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol Psychiatr 1997; 41: 636–43
Eastwood SL, McDonald B, Burnet PW, et al. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Mol Brain Res 1995; 29: 211–23
Sokolov BP. Expression of NMDAR1, GluRl, GluR7, and KA1 glutamate receptor mRNAs is decreased in frontal cortex of “neuroleptic-free” schizophrenics: evidence on reversible upregulation by typical neuroleptics. J Neurochem 1998; 71: 2454–64
Chen ACH, Kalsi G, Brynjolfsson J, et al. Exclusion of linkage of schizophrenia to the gene for the glutamate GluR5 receptor. Biol Psychiatry 1997; 41: 243–5
Chen ACH, Kalsi G, Brynjolfsson J, et al. Lack of evidence for close linkage of the glutamate GluR6 receptor gene with schizophrenia. Am J Psychiatry 1996; 153: 1634–6
Eastwood SL, Porter RHP, Harrison PJ. The effect of chronic haloperidol treatment on glutamate receptor subunit (GluR1, GluR2, KA 1, KA2, NR1) mRNAs and glutamate binding protein mRNA in rat forebrain. Neurosci Lett 1996; 212: 163–6
Fitzgerald LW, Deutch AY, Gasic G, et al. Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J Neurosci 1995; 15: 2453–61
Healy DJ, Meador-Woodruff JH. Clozapine and haloperidol differentially affect AMPA and kainate receptor subunit mRNA levels in rat cortex and striatum. Mol Brain Res 1997; 47: 331–8
McCoy L, Cox C, Richfield EK. Chronic treatment with typical and atypical antipsychotics increases the AMPA-preferring form of AMPA receptor in rat brain. Eur J Pharmacol 1996; 318: 41–5
McCoy L, Cox C, Richfield EK. Antipsychotic drug regulation of AMPA receptor affinity states and GluR1, GluR2 splice variant expression. Synapse 1998; 28: 195–207
Meador-Woodruff JH, King RE, Damask SP, et al. Differential regulation of hippocampal AMPA and kainate receptor sub-unit expression by haloperidol and clozapine. Mol Psychiatry 1996; 1: 41–53
Ossowska K, Pietraszek M, Wardas J. Further evidence for the subsensitivity of striatal AMPA receptors, induced by chronic haloperidol administration — an autoradiographic study. N-S Arch Pharmacol 1996; 354: 384–8
Tarazi FI, Florijn WJ, Creese I. Regulation of ionotropic glutamate receptors following subchronic and chronic treatment with typical and atypical antipsychotics. Psychopharmacology 1996; 128: 371–9
Tascedda F, Lovati E, Blom JM, et al. Regulation of ionotropic glutamate receptors in the rat brain in response to the atypical antipsychotic Seroquel (quetiapine fumarate). Neuropsycho-pharmacol 1999; 21: 211–7
Spurney CF, Baca SM, Murray AM, et al. Differential effects of haloperidol and clozapine on ionotropic glutamate receptors in rats. Synapse 1999; 34: 266–76
Johnson SA, Luu NT, Herbst TA, et al. Synergistic interactions between ampakines and antipsychotic drugs. J Pharmacol ExpTher 1999; 289: 392–7
Granger R, Deadwyler S, Davis M, et al. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse 1996; 22: 332–7
Thompson DM, Guidotti A, Dibella M, et al. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proc Natl Acad Sci U S A 1995; 92: 7667–71
Uzunov DP, Zivkovich I, Pirkle WH, et al. Enantiomeric resolution with a new chiral stationary phase of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide, a cognition-enhancing benzothiadiazine derivative. J Pharm Sci 1995; 84: 937–42
Zivkovic I, Thompson DM, Bertolino M, et al. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-α-amino-2,3-dihydro-5-methyl-3-oxo-4-lsoxazolepropanoic acid (AMPA) receptor desensitization. J Pharmacol Exp Ther 1995; 272: 300–9
Yamada KA. Modulating excitatory synaptic neurotransmission: potential treatment for neurological disease? Neurobiol Dis 1998; 5: 67–80
Lutfy K, Cai SX, Woodward RM, et al. Antinociceptive effects of NMDA and non-NMDA receptor antagonists in the tail flick test in mice. Pain 1997; 70: 31–40
Nishiyama T, Gyermek L, Lee C, et al. The spinal antinociceptive effects of a novel competitive AMPA receptor antagonist, YM872, on thermal or formalin-induced pain in rats. Anesth Analg 1999; 89: 143–7
Nishiyama T, Gyermek L, Lee C, et al. The systemically administered competitive AMPA receptor antagonist, YM872, has analgesic effects on thermal or formalin-induced pain in rats. Anesth Analg 1999; 89: 1534–7
Szekely JI, Kedves R, Mate I, et al. Apparent antinociceptive and anti-inflammatory effects of GYKI 52466. Eur J Pharmacol 1997; 336: 143–54
McLemore GL, Kest B, Inturrisi CE. The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res 1997; 778: 120–6
Kest B, McLemore G, Kao B, et al. The competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. J Pharmacol Exp Ther 1997; 283: 1249–55
Nishiyama T, Gyermek L, Lee C, et al. Analgesic interaction between intrathecal midazolam and glutamate receptor antagonists on thermal-induced pain in rats. Anesthesiology 1999; 91: 531–7
Harris JA, Corsi M, Quartaroli M, et al. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience 1996; 74: 7–12
Sorkin LS, Yaksh TL, Doom CM. Mechanical allodynia in rats is blocked by a Ca2+ permeable AMPA receptor antagonist. NeuroReport 1999; 10: 3523–6
Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res 1999; 820: 63–70
Okano K, Kuraishi Y, Satoh M. Involvement of spinal substance P and excitatory amino acids in inflammatory hyperalgesia in rats. Jpn J Pharmacol 1998; 76: 15–22
Stanfa LC, Dickenson AH. The role of non-N-methyl-D-aspartate ionotropic glutamate receptors in the spinal transmission of nociception in normal animals and animals with carrageenan inflammation. Neuroscience 1999; 93: 1391–8
Hunter JC, Singh L. Role of excitatory amino acids in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett 1994; 174: 217–21
Simmons RMA, Li DL, Hoo KH, et al. Kainate GluR5 subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology 1998; 37: 25–36
Li P, Wilding TJ, Kim SJ, et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 1999; 397: 161–4
Kotlinska J, Liljequist S. The putative AMPA receptor agonist, LY326325, produces anxiolytic-like effects without altering locomotor activity in rats. Pharmacol Biochem Behav 1998; 60: 119–24
Mathe JM, Fagerquist MV, Svensson TH. Antipsychotic-like effect of the AMPA recptor antagonist LY326325 as indicated by suppression of conditioned avoidance response in the rat. J Neural Transm 1999; 106: 1003–9
Czlonkowska A, Siemiatkowski M, Plaznik A. Some behavioral effects of AMPA/kainate receptor agonists and antagonists. J Physiol Pharmacol 1997; 48: 479–88
Rasmussen K, Kendrick WT, Kogan JH, et al. A selective AMPA antagonist, LY293558 suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacol 1996; 15: 497–505
Schmidt WJ, Kretschmer BD. Behavioural pharmacology of glutamate receptors in the basal ganglia. Neurosci Biobehav Rev 1997; 21: 381–92
Starr MS, Starr BS, Kaur S. Stimulation of basal and L-DOPA-induced motor activity by glutamate antagonists in animal models of Parkinson’s disease. Neurosci Biobehav Rev 1997; 21: 437–46
Ehrenberger K, Felix D. Caroverine depresses the activity of cochlear glutamate receptors in guinea pigs: invivomodel for drug-induced neuroprotection. Neuropharmacology 1992; 31: 1259–63
Denk DM, Heinzl H, Franz P, et al. Caroverine in tinnitus treatment: a placebo-controlled blind study. Acta Oto-Laryngologica 1997; 117: 825–30
Bruyn RP, Stoof JC. The quinolinic acid hypothesis in Huntington’s chorea. J Neurol Sci 1990; 95: 29–38
Rubinsztein DC, Leggo J, Chiano M, et al. Genotypes at the GluR6 kainate receptor locus are associated with variation in the age of onset of Huntington disease. Proc Natl Acad Sci USA 1997; 94: 3872–6
MacDonald ME, Vonsattel JP, Shrinidhi J, et al. Evidence for the GluR6 gene associated with younger onset age of Huntington’s disease. Neurology 1999; 53: 1330–2
Gahring LC, Rogers SW, Twyman RE. Autoantibodies to glutamate receptor subunit GluR2 in nonfamilial olivopontocerebellar degeneration. Neurology 1997; 48: 494–500
Smith T, Groom A, Zhu B, et al. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nature Med 2000; 6: 62–6
Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nature Med 2000; 6(1): 67–70
Morrison BM, Morrison JH. Amyotrophic lateral sclerosis associated with mutations in Superoxide dismutase: a putative mechanism of degeneration. Brain Res Rev 1999; 29: 121–35
Rothstein JD. Excitotoxicity and neurodegeneration in amyo-trophic lateral sclerosis. Clin Neurosci 1995-96; 3: 348–59
Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol 1997; 244 Suppl 2: S3–S14
Canton T, Pratt J, Stutzmann JM, et al. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. NeuroReport 1998; 9: 775–8
Roy J, Minotti S, Dong L, et al. Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J. Neurosci 1998; 18: 9673–84
Morrison BM, Janssen WGM, Gordon JW, et al. Light and electron microscopic distribution of the AMPA receptor subunit, GluR2, in the spinal cord of control and G86R mutant superoxide dismutase transgenic mice. J Comp Neurol 1998; 395: 523–34
Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci 1996; 16: 4069–79
Ikonomidou C, Qin Qin Y, Labruyere J, et al. Motor neuron degeneration induced by excitotoxin agonists has features in common with those seen in the SOD-1 transgenic mouse model of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 1996; 55: 211–24
Terro F, Yardin C, Esclaire F, et al. Mild kainate toxicity produces selective motoneuron death with marked activation of Ca2+-permeable AMPA/kainate receptors. Brain Res 1998; 809: 319–24
Fryer HJ, Knox RJ, Strittmatter SM, et al. Excitotoxic death of a subset of embryonic rat motor neurons invitro. J Neurochem 1999; 72: 500–13
Arias C, Becerra-Garcia F, Tapia R. Glutamic acid and Alzheimer’s disease. Neurobiology 1998; 6: 33–43
Olney JW, Wozniak DF, Farber NB. Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies. Arch Neurol 1997; 54: 1234–40
Mattson MP. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 1997; 77: 1081–132
Kirazov L, Loffler T, Schliebs R, et al. Glutamate-stimulated secretion of amyloid precursor protein from cortical rat brain slices. Neurochem Int 1997; 30: 557–63
Ulus IH, Wurtman RJ. Metabotropic glutamate receptor agonists increase release of soluble amyloid precursor protein derivatives from rat brain cortical and hippocampal slices. J Pharmacol Exp Ther 1997; 281: 149–54
Kirson ED, Yaari Y, Perouansky M. Presynaptic and postsynaptic actions of halothane at glutamatergic synapses in the mouse hippocampus. BrJ Pharmacol 1998; 124: 1607–14
Minami K, Wick MJ, Stern-Bach Y, et al. Sites of volatile anesthetic action on kainate (Glutamate receptor 6) receptors. J Biol Chem 1998; 273: 8248–55
Kamiya Y, Andoh T, Furuya R, et al. Comparison of the effects of convulsant and depressant barbiturate stereoisomers on AMPA-type glutamate receptors. Anesthesiology 1999; 90: 1704–13
Acknowledgements
Part of the research reported in this review was supported by the Health Research Council of New Zealand, the Neurological Foundation of New Zealand, and the Auckland Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lees, G.J. Pharmacology of AMPA/Kainate Receptor Ligands and Their Therapeutic Potential in Neurological and Psychiatric Disorders. Drugs 59, 33–78 (2000). https://doi.org/10.2165/00003495-200059010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059010-00004