Abstract
-
▴ Capecitabine is an orally administered fluoropyrim-idine carbamate used for the treatment of paclitaxel- or anthracycline-refractory breast cancer.
-
▴ Capecitabine is metabolised via a 3-step process to the active agent fluorouracil. The final step of this process occurs preferentially in malignant tissue.
-
▴ In patients with paclitaxel-refractory breast cancer receiving capecitabine (2510 mg/m2 /day for 2 weeks of a 3-week cycle) the objective tumour response rate was 20%. Disease progression occurred in 34% of patients and 40% had stable disease.
-
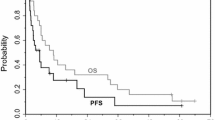
▴ In this trial, the median duration of response was 241 days. Disease progression or death occurred in 83% of patients, and median time to progression was 93 days. Median survival time was 384 days.
-
▴ In previously untreated patients with breast cancer, the response rate was higher and time to disease progression was longer after oral capecitabine (2510 mg/m2 /day for 2 weeks of a 3-week cycle) than after intravenous cyclophosphamide, methotrexate and fluorouracil therapy.
-
▴ In clinical trials, generally gastrointestinal or haematological adverse events were reported most frequently. Other commonly reported events included hand-and-foot syndrome, fatigue, hyperbilirubinaemia, dermatitis and anorexia.
Similar content being viewed by others
References
Roche Laboratories Inc. Xeloda (capecitabine) prescribing information. USA, 1998
Frings S. Capecitabine: a novel oral tumor-activated fluoro-pyrimidine. Onkologie 1998; 21: 451–8
Bunnell C, Winer E. Oral 5-FU analogues in the treatment of breast cancer. Oncology 1998; 12 (10 Suppl. 7): 39–43
Pazdur R, Hoff P, Medgyesy D, et al. The oral fluouracil prodrugs. Oncology 1998; 12 (10 Suppl. 7): 48–51
Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoro-pyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998; 34(8): 1274–81
Ishikawa T, Fukase Y, Yamamoto T, et al. Antitumor activities of a novel fluoropyrimidine, N4-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine (capecitabine). Biol Pharm Bull 1998; 21(7): 713–7
Ishikawa T, Sekiguchi F, Fukase Y, et al. Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehy-drogenase activities in tumors in human cancer xenografts. Cancer Res 1998 Feb 15; 58: 685–90
Sawada N, Ishikawa T, Fukase Y, et al. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 1998 Apr; 4: 1013–9
Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 1998; 55: 1091–7
Budman DR, Meropol NJ, Reigner B, et al. Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 1998 May; 16(5): 1795–802
Mackean M, Planting A, Twelves C, et al. Phase I and pharmalogic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 1998; 16(9): 2977–85
Cassidy J, Dirix L, Bissett D, et al. Aphase I study of capecitabine in combination with oral leucovorin in patients with intractable solid tumors. Clin Cancer Res 1998; 4: 2755–61
Schüller J, Cassidy J, Reigner B, et al. Tumor selective activation of capecitabine in colorectal cancer patients [abstract]. Onkologie 1997 Oct; 20 Suppl. 1: 189
Blum J, Jones S, Buzdar A, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999 Feb; 17(2): 485–93
O’Reilly SM, Moiseyenko V, Talbot DC. A randomized phase II study of Xeloda™ (capecitabine) vs paclitaxel in breast cancer patients failing previous anthracycline therapy [abstract]. 34th Proc Am Soc Clin Oncol 1998 May 16; 17: 163a
O’Shaughnessy J, Moiseyenko V, Bell D, et al. A randomized phase II study of Xeloda™ (capecitabine) vs CMF as first line chemotherapy of breast cancer in women aged greater than or equal to 55 years [abstract]. 34th Proc Am Soc Clin Oncol 1998 May 16; 17: 103a
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dooley, M., Goa, K.L. Capecitabine. Drugs 58, 69–76 (1999). https://doi.org/10.2165/00003495-199958010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958010-00006