Abstract
-
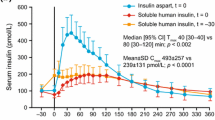
▴ Insulin aspart is a recombinant analogue of human insulin. Following subcutaneous insulin injection (0.15 to 0.2 U/kg), significantly higher serum insulin concentrations are achieved in a shorter time with insulin aspart than with human insulin. The subsequent decline in serum insulin concentrations is also more rapid with insulin aspart.
-
▴ In healthy individuals undergoing euglycaemic glucose clamp testing, glucose infusion rates were higher and reached maximum concentrations significantly earlier after insulin aspart than after human insulin.
-
▴ Interindividual variability in pharmacodynamic and pharmacokinetic parameters with insulin aspart was generally less than that with human insulin, whereas the intraindividual variability in these parameters was similar after each insulin.
-
▴ In patients with type 1 diabetes postprandial glucose excursions were less pronounced with insulin aspart than human insulin. Daytime glucose control was better and minimum glucose levels during the night were not as low with insulin aspart as with human insulin.
-
▴ In diabetic patients treated with insulin aspart there was generally a lower frequency of hypoglycaemic events than in patients treated with human insulin.
Similar content being viewed by others
References
Drejer K, Kruse V, Larsen UD, et al. Receptor binding and tyrosine kinase activation by insulin analogues with extreme affinities studied in human hepatoma HepG2 cells. Diabetes 1991 Nov; 40: 1488–95
Volund A, Brange J, Drejer K, et al. In vitro and in vivo potency of insulin analogues designed for clinical use. Diabet Med 1991 Nov; 8: 839–47
Bornfeldt KE, Gidlöf RA, Wasteson A, et al. Binding and biological effects of insulin, insulin analogues and insulin-like growth factors in rat aortic smooth muscle cells. Comparison of maximal growth promoting activities. Diabetologia 1991 May; 34: 307–13
Drejer K. Bioactivity of insulin analogues. In: Berger M, Gries FA, editors. Frontiers in insulin pharmacology: international symposium, Hamburg. Stuttgart: Georg Thieme Verlag, 1993: 17–27
Heinemann L, Heise T, Jorgensen LN, et al. Action profile of the rapid acting insulin analogue: human insulin B28Asp. Diabet Med 1993 Jul; 10: 535–9
Heinemann L, Kapitza C, Starke AAR, et al. Time-action profile of the insulin analogue B28Asp. Diabetic Med 1996 Jul; 13: 683–4
Heinemann L, Weyer C, Rave K, et al. Comparison of the time-action profiles of U40- and U100-regular human insulin and the rapid-acting insulin analogue B28 Asp. Exp Clin Endocrinol Diabetes 1997; 105: 140–4
Heinemann L, Weyer C, Rauhaus M. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 1998 Nov; 21(11): 1910–4
Home P, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol (In press)
Kang S, Brange J, Burch A, et al. Absorption kinetics and action profiles of subcutaneously administered insulin analogues (AspB9GluB27, AspB10, AspB28) in healthy subjects. Diabetes Care 1991 Nov; 14: 1057–65
Lindholm A, McEwen J, Riis A. Significantly improved postprandial glycaemic control with the novel rapid-acting insulin aspart [abstract]. Diabetologia 1998 Aug; 41 Suppl. 1: A49
Wiefels K, Hübinger A, Dannehl K, et al. Insulinkinetic and-dynamic in diabetic patients under insulin pump therapy after injections of human insulin or the insulin analogue (B28Asp). Horm Metab Res 1995; 27: 421–4
Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10, AspB28) on meal-related plasma glucose excursions in type I diabetic subjects. Diabetes Care 1991 Jul; 14: 571–7
Rosenfalck AM, Thorsby P, Kjems L, et al. Effects of the rapid-acting insulin analogue insulin aspart on postprandial glycaemic excursions compared to human soluble insulin actrapid given immediately or 30 minutes before a meal in insulin treated type 2 diabetes patients. Acta Diabetologica [abstract]. 1998; 35(4): 246
Lutterman J, Pijpers E, Netten P, et al. Glycaemic control in IDDM patients during one day with injection of human insulin or the insulin analogues insulin X14 and insulin X14(+Zn). In: Berger M, Gries F, editors. Frontiers in insulin pharmacology: international symposium, Hamburg. Stuttgart: Thieme Medical Publishers, 1993: 102–9
Jensen I, Kruse V, Larsen UD. Scintigraphic studies in rats: kinetics of insulin analogues covering wide range of receptor affinities. Diabetes 1991; 40(5): 628–32
Home P, Lindholm A, Hylleberg B. Improved glycemic control with insulin aspart — a multicenter randomized double-blind crossover trial in type 1 diabetic patients. Diabetes Care 1998 Nov; 21(11): 1904–9
Ewing FME, Frier BM. Comparison of the physiological and symptomatic responses to hypoglycaemia induced by human soluble insulin and the insulin analogue aspart in patients with type 1 diabetes [abstract]. Diabetic Med 1998; 15 Suppl. 2: S34
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simpson, K.L., Spencer, C.M. Insulin Aspart. Drugs 57, 759–765 (1999). https://doi.org/10.2165/00003495-199957050-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199957050-00013