Summary
Vinca alkaloids, including vinblastine, vincristine, vindesine and vinorelbine, are widely used antineoplastic drugs, either as single agents or in combination with other drugs. The mechanism of action of these cell cycle-dependent agents is the inhibition of tubulin polymerisation into microtubules. Numerous studies have been conducted in animals and humans, using various in vivo and in vitro models, to investigate the pharmacological behaviour of this class of antitumour drug.
Studies in cellular pharmacology demonstrate that vinca alkaloids are transported by multiple mechanisms, including passive diffusion and energy- and temperature-dependent active transport systems. Moreover, active efflux of drug is involved in the P-glycoprotein-mediated multidrug resistance to vinca alkaloids. This phenomenon may be modulated, in vivo and in vitro, by calcium antagonists and calmodulin inhibitors.
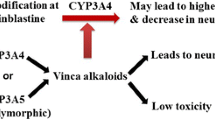
The clinical pharmacokinetics of vinca alkaloids after intravenous bolus injection, continuous infusion and oral administration are characterised by a large apparent total volume of distribution, high total plasma clearance and long terminal elimination half-life. Biliary excretion is the main elimination pathway, with low urinary excretion. Pharmacokinetic parameters of vinca alkaloids are time- and dose-dependent, and large inter- and intra-individual variabilities have been observed. Human hepatic P-450IIIA cytochromes are involved in the metabolism of vindesine, vinblastine and probably other vinca alkaloids. Therefore, the possibility of drug-drug interactions must be considered when coadministering drugs in combination cancer chemotherapy.
Development of newer semisynthetic analogues of vinca alkaloids and conjugation of vinca alkaloids with monoclonal antibodies may result in derivatives with increased antitumour activity and less clinical toxicity.
Similar content being viewed by others
References
Bech-Hansen NT, Till JE, Ling V. Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. Journal of Cell Physiology 88: 23–32, 1976
Beck WT. Vinca-alkaloid resistant phenotype in cultured human leukemic lymphoblasts. Cancer Treatment Reports 67: 875–882, 1983
Beck WT, Cirtain MC. Continued expression of vinca alkaloid resistance by CCRF-CEM cells after treatment with tunicamycin or pronase. Cancer Research 42: 184–189, 1992
Beck WT, Mueller TJ, Tanzer LR. Altered surface membrane glycoproteins in vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Research 39: 2070–2076, 1979
Beck WT, Danks MK, Cirtain MC, van Heiningen JN. Cross-resistance patterns and antigene expression in vinca alkaloid-and other multi drug-resistant human leukemic cell lines. Progress in Clinical and Biological Research 233: 2333–2340, 1986
Biedler JL, Peterson RHF. Altered plasma membrane glycocon-jugates of Chinese hamster cells with acquired resistance to actinomycin D, daunorubicin and vincristine. In Sartorelli et al. (Eds) Molecular actions and targets for cancer chemotherapeutic agents, pp. 453–482, Academy Press, New York, 1981
Binet S, Chineau E, Fellous A, Lataste H, Krikorian A, et al. Immunofluorescence study of the action of navelbine, vincristine and vinblastine on mitotic and axonal microtubules. International Journal of Cancer 46: 262–266, 1990
Bleyer WA, Frisby SA, Oliverio VT. Uptake and binding of vincristine by murine leukemia cells. Biochemical Pharmacology 24: 633–639, 1975
Bloemhof H, Van Jijk KN, De Graaf SSN, Vendrig DEMM, Uges DRA. Sensitive method for the determination of vincristine in human serum by high-performance liquid chromatography after on-line column-extraction. Journal of Chromatography - Biomedical Applications 572: 171–179, 1991
Bodey GP, Yap HY, Yap BS, Valdivieso M. Continuous infusion of vindesine in solid tumors. Cancer Treatment Reviews 7: 39–47, 1980
Bore P, Rahmani R, van Cantfort J, Focan C, Cano JP. Pharmacokinetics of a new anticancer drug, navelbine, in patients: comparative study of radioimmunologic and radioactive determination methods. Cancer Chemotherapy and Pharmacology 23: 247–251, 1989
Bosmann HB. Mechanism of cellular drug resistance. Nature 233: 566–569, 1971
Bowman LC, Houghton JA, Houghton PJ. GTP influences the binding of vincristine in human tumor cytosols. Biochemical and Biophysical Research Communications 135: 695–700, 1986
Brade WP. Critical review of pharmacology, toxicology, pharmacokinetics of vincristine, vindesine, vinblastine. Beiträge zur Onkologie 6. Proceedings of the International Vincaalkaloïd Symposium — Vindesine: 95-123, 1981
Budman DR, Shulman P, Marks M, Vinciguerra V, Weiselberg L, et al. Phase I trial of vinzolidine. Cancer Treatment Reports 68: 979–982, 1984
Cano JP, Rahmani R, Fabre G, Richard B, Lacarelle B, et al. Human hepatocytes as an alternative model to the use of animal in experiments. In Guillouzo (Ed.) Liver cells drugs, Vol. 164, pp. 301-307, Colloque INSERM/John Libbey Eurotext Ltd, 1988
Cantwell BM, Bozzino JM, Corris P, Harris AL. The multidrug resistant phenotype in clinical practice: evaluation of cross resistance to ifosfamide and mesna after VP 16-213, doxorubicin and vincristine (VPAV) for small cell lung cancer. European Journal of Cancer and Clinical Oncology 24: 123–129, 1988
Carlsen SA, Till JE, Ling V. Modulation of membrane drug per- meability in Chinese hamster ovary cells. Biochimica Biophysica Acta 455: 900–912, 1976
Cersosimo RJ, Bromer R, Licciardello JTW, Ki H. Pharmacology, clinical efficacy and adverse effects of vindesine sulfate, a new vincaalkaloid. Pharmacotherapy 3: 259–274, 1983
Cros S, Wright M, Morimoto M, Lataste H, Couzinier JP, et al. Experimental antitumour activity of navelbine. Seminars in Oncology 16 (Suppl. 4): 15–20, 1989
Dalton WS, Durie BGM, Alberts DS, Gerlach JH, Gress AG. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Research 46: 5125–5130, 1986
Danks MK, Metzger DW, Ashmun RA, Beck WT. Monoclonal antibodies to glycoproteins of vinca alkaloid-resistant human leukemic cells. Cancer Research 45: 3220–3224, 1985
Dano K. Active outward transport of daunomycih in resistant Ehrlich ascites tumor cells. Biochimica Biophysica Acta 323: 466–483, 1973
De Bruijn MHL, Van Der Bliek AM, Biedler JL, Borst P. Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Molecular and Cellular Biology 6: 4717–4722, 1986
De Smet M, Van Belle SJP, Storme GA, Massart DL. High performance liquid Chromatographic determination of vinca alkaloids in plasma and urine. Journal of Chromatography - Biomedical Applications 345: 309–321, 1985
Ferguson PJ, Cass CE. Differential cellular retention of vincristine and vinblastine by cultured human promyelocytic leukemia HL-60/CI cells/the basis of differential toxicity. Cancer Research 45: 5480–5488, 1985
Ferguson PJ, Phillips JA, Seiner M, Cass CE. Differential activity of vincristine and vinblastine against cultured cells. Cancer Research 44: 3307–3312, 1984
Focan C, Mazy V, Lévi F, Bruguerolle B, Zhou XJ, et al. Circadian-varying plasma concentration of vindesine during 48-hour continuous infusion at a constant rate. In Ensminger et al. (Eds) Update in drug delivery systems, pp. 291–285, Futura Publishing Co., Inc., Mount Kisco, NY, 1989
Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacological Reviews 42: 155–199, 1990
Fry DC, Kuby SA, Mildvan AS. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, Fl-ATPase, and other nucleotide-binding proteins. Proceedings of the National Academy of Sciences of United States of America 83: 907–911, 1986
Haber H, Norris MD, Kavallaris M, Bell DR, Davey RA, et al. Atypical multidrug resistance in a therapy-induced drug-resistant human leukaemic cell line (LALW-2) resistance to vinca alkaloids independent of P-glycoprotein. Cancer Research 49: 5281–5287, 1989
Hacker MP, Dank JR, Ershler WB. Vinblastine pharmacokinetics measured by a sensitive enzyme-linked immunosorbent assay. Cancer Research 44: 478–481, 1984
Hande K, Gay J, Gober J, Greco FA. Toxicity and pharmacology of bolus vindesine injection and prolonged vindesine infusion. Cancer Treatment Reviews 7: 25–31, 1980
Higgins CF, Hiles ID, Salmond GPC, Gill DR, Downie JA, et al. A family of related ATP-binding subunits coupled to many biological processes in bacteria. Nature 323: 448–450, 1986
Houghton PJ, Houghton JA, Bowman LC, Hazelton BJ. Therapeutic selectivity of vinca alkaloids: a role for guanosine 5′- triphosphate. Anti-Cancer Drug Design 2: 165–179, 1987
Huhtikangas A, Lehtola T, Lapinjoki S, Lounasmaa M. Specific radioimmunoassay for vincristine. Planta Medica 53: 85–87, 1987
Inaba M, Johnson RK. Decreased retention of actinomycin D as the basis for cross-resistance in anthracycline-resistant sublines of P388 leukemia. Cancer Research 37: 4629–4634, 1977
Jackson DV, Sethi VS, Spurr CL, White DR, Richard FI, et al. Pharmacokinetics of vincristine infusion. Cancer Treatment Reports 65: 1043–1048, 1981
Jehl F, Debs J, Herlin C, Quiox E, Caillon C, et al. Determination of navelbine and desacetylnavelbine in biological fluids by highperformance liquid chromatography. Journal of Chromatography — Biomedical Applications 525: 225–233, 1990
Jehl F, Quiox E, Leveque D, Pauli C, Breillout F, et al. Phar- macokinetics and preliminary metabolic fate of navelbine in humans as determined by high performance liquid chromatography. Cancer Research 51: 2073–2076, 1991
Johnson IS, Armstrong JG, Gorman M, Burnett JP. The vinca alkaloids: a new class of oncolytic agents. Cancer Research 23: 1390–1427, 1963
Jordan MA, Himes RH, Wilson L. Comparison of the effects of vinblastine, vincristine, vindesine and vinepidine on microtubule dynamics and cell proliferation in vitro. Cancer Research 45: 2741–2747, 1985
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica Biophysica Acta 455: 152–162, 1976
Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221: 1285–1288, 1983
Lapinjoki SP, Veräjänkorva HM, Huhtikangas AE, Lehtola TJ, Lounasmaa M. An enzyme-linked immunosorbent assay for the antineoplastic agent vincristine. Journal of Immunoassay 7: 113–128, 1986
Lazuzza BC, Nichols CL, Brrigs SL, Cullinan GJ, Johnson DA, et al. New antitumor monoclonal antibody-vinca conjugates LY203725 and related compounds: design, preparation, and representative in vivo activity. Journal of Medicinal Chemistry 32: 548–555, 1989
Legha SS. Vincristine neurotoxicity: pathophysiology and management. Medical Toxicology 1: 421–427, 1986
Ling V, Kartner N, Sudo T, Siminovitch L, Riordan JR. Multi-drug-resistance phenotype in Chinese hamster ovary cells. Cancer Treatment Reports 67: 869–874, 1983
Lothstein L, Hsu SIH, Horwitz SB, Greenberger LM. Alternate overexpression of two phosphoglycoprotein genes is associated with changes in multidrug resistance in a J774.2 cell line. Journal of Biological Chemistry 264: 16054–16058, 1989
Louie KG, Hamilton TC, Winker MA, Behrens BC, Tsuruo T, et al. Adriamycin accumulation and metabolism in adriamy- cin-sensitive and resistant human ovarian cancer cells lines. Biochemical Pharmacology 35: 467–472, 1986
Lu K, Yap HY, Loo TL. Clinical pharmacokinetics of vinblastine by continuous intravenous infusion. Cancer Research 43: 1405–1408, 1983
Mangeney P, Adriamialisoa RZ, Langlois N, Langlois Y, Potier P. A new class of antitumor compounds: 5′ nor and 5′, 6 seco derivatives of vinblastine-type alkaloids. Journal of Organic Chemistry 44: 3765–3768, 1979
Maral R, Bourut C, Chenu E, Mathé G. Experimental in vivo cross-resistance of vinca alkaloid drugs. Cancer Chemotherapy and Pharmacology 5: 197–199, 1981
Maral R, Bourut C, Chenu E, Mathé G. Experimental antitumor activity of 5′-nor-anhydrovinblastine, navelbine. Cancer Letters 22: 49–54, 1984
Marshall E, Spearman R, Goodwin M, Kau D. Disposition of the monoclonal antibody-vinca alkaloid conjugate, KS1/2-DAVLB (LY 256787), in Fisher 344 rats and rhesus monkeys. Drug Metabolism and Disposition 15: 640–647, 1987
Mathé G, Hulhoven R, Sokal G, Bayssas M, Belpomme D, et al. Phase II clinical trials with vindesine in patients with héma- tologie malignancies. Anticancer Research 1: 1–9, 1981
Nelson RL. The comparative clinical pharmacology and pharmacokinetics of vindesine, vincristine and vinblastine in human patients with cancer. Medical and Pediatric Oncology 10: 115–127, 1982
Owellen RJ, Donigian DW, Hartke CA, Hains FO. Correlation of biologic data with physicochemical properties among the vinca alkaloids and their congeners. Biochemical Pharmacology 26: 1213–1219, 1977a
Owellen RJ, Hartke CA, Hains FO. Pharmacokinetics and metabolism of vinblastine in humans. Cancer Research 37: 2597–2602, 1977b
Owellen RJ, Owens AH, Donigian DW. The binding of vinblastine, vincristine and colchicine to tubulin. Biochemical Biophysical Research Communications 47: 685–691, 1972
Pierré A, Lavielle G, Hautefaye P, Seurre G, Leonce S, et al. Pharmacological properties of a new α-aminophosphonic acid derivative of vinblastine. Anticancer Research 10: 139–144, 1990
Pontarotti PA, Rahmani R, Martin M, Barbet M, Barbet J. Monoclonal antibodies to antitumor vinca alkaloids: thermodynamics and kinetics. Molecular Immunology 22: 227–285, 1985
Rahmani R, Barbet J, Cano JP. A 125I-radiolabelled probe for vinblastine and vindesine radioimmunoassays. Clinica Chimica Acta 129: 57–69, 1983
Rahmani R, Bruno R, Fabre G, Cano JP. Extrapolation of preclinical pharmacokinetic data to therapeutic drug use. Xenobiotica 18 (Suppl. 1): 71–88, 1988
Rahmani R, Guéritte F, Martin M, Just S, Cano JP, et al. Comparative pharmacokinetics of antitumor vinca alkaloids: intra- venous bolus injections of navelbine and related alkaloids to cancer patients and rats. Cancer Chemotherapy and Pharmacology 16: 223–228, 1986
Rahmani B, Kleisbauer JP, Cano JP, Martin M, Barbet J. Clinical pharmacokinetics of vindesine infusion. Cancer Treatment Report 69: 839–844, 1985
Rahmani R, Martin M, Barbet J, Cano JP. Radioimmunoassay and preliminary pharmacokinetic studies in rats of 5’-noran- hydrovinblastine (navelbine). Cancer Research 44: 5609–5613, 1984a
Rahmani R, Martin M, Favre R, Cano JP, Barbet J. Clinical pharmacokinetics of vindesine: repeated treatments by intra- venous bolus injections. European Journal of Cancer and Clinical Oncology 29: 1400–1417, 1984b
Rahmani R, Bruno R, Iliadis A, Favre R, Just S, et al. Clinical pharmacokinetics of the antitumor drug navelbine (5’-noran- hydrovinblastine). Cancer Research 47: 5796–5799, 1987
Rahmani R, Zhou XJ, Bore P, Van Cantfort J, Focan C, et al. Oral administration of [3H]navelbine in patients: comparative pharmacokinetics using radioactive and radioimmunologic determination methods. Anti-Cancer Drugs 2: 405–410, 1991
Rahmani R, Zhou XJ, Placidi M, Cano JP. In vivo and in vitro pharmacokinetics and metabolism of vinca alkaloids in rat. I. Vindesine (4-desacetyl-vinblastine 3-carboxyamide). European Journal of Drug Metabolism and Pharmacokinetics 15: 49–55, 1990
Rahmani-Jourdheuil D, Placidi M, Carcassonne Y, Rahmani R. Uptake of navelbine, a novel antitumor drug, by rat liver plasma membrane vesicles: evidence of passive diffusion and active transport system. Journal of Cellular Pharmacology 2: 9–17, 1991
Rao KSPB, Collard MP, Trouet A. Vinca-23-oyl amino acid de- rivatives: as new anticancer agents. Anticancer Research 5: 379–386, 1985
Ratain MJ, Vogelzang NJ. Phase I and pharmacological study of vinblastine by prolonged continuous infusion. Cancer Research 46: 4827–4830, 1986
Ratain MJ, Vogelzang NJ, Sinkule JA. Interpatient and intra- patient variability in vinblastine pharmacokinetics. Clinical Pharmacology and Therapeutics 41: 61–67, 1987
Retsas S, Newton KA, Westbury G. Vinca alkaloids and platelets. New England Journal of Medicine 299: 310, 1978
Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, et al. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 316: 817–819, 1985
Sethi VS, Burton SS, Jackson D. A sensitive radioimmunoassay for vincristine and vinblastine. Cancer Chemotherapy and Pharmacology 4: 183–187, 1980
Singer WD, Hirnes RH. Cellular uptake and tubulin binding properties of four vinca alkaloids. Biochemical Pharmacology 43: 545–551, 1992
Spearman ME, Goodwin RM, Apelgren LD, Bumol TF. Disposition of the monoclonal antibody-vinca alkaloid conjugate KS1/4-DAVLB (LY256787) and free 4-desacetylvinblastine (DAVLB) in tumor-bearing nude mice. Journal of Pharmacology and Experimental Therapeutics 241: 695–703, 1987
Todd GC, Gibson WR, Morton DM. Toxicology of vindesine (deacetyl vinblastine amide) in mice, rats and dogs. Journal of Toxicology and Environmental Health 1: 843–850, 1976
Tsuruo T, Lida H, Tsukagoshi S, Sakurai Y. Overcoming of vin- cristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by vera- pamil. Cancer Research 41: 1967–1972, 1981
Tsuruo T, Lida H, Tsukagoshi S, Sakurai Y. Potentiation of vin- cristine and adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Research 43: 2267–2272, 1983
Van den Berg HW, Desai ZR, Wilson R, Kennedy G, Bridges JM, et al. The pharmacokinetics of vincristine in man: reduced drug clearance associated with raised serum alkaline phosphatase and dose-limited elimination. Cancer Chemotherapy and Pharmacology 8: 215–219, 1982
Van der Blick AM, Van der Velde-Koerts T, Ling V, Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Molecular and Cellular Biology 6: 1671–1678, 1986
Vendrig DEMM, Hothuis JJM, Erdélyi-Toth V, Hulshoff A. Solid phase extraction of vinblastine and vincristine from plasma and urine: variable drug recoveries due to non-reproducible column packings. Journal of Chromatography — Biomedical Applications 414: 91–100, 1987
Vendrig DEMM, Teeuwsen J, Hothuis JJM. Analysis of vinca alkaloids in plasma and urine using high performance liquid chromatography with electro-chemical detection. Journal of Chromatography — Biomedical Applications 424: 83–94, 1988a
Vendrig DEMM, Teeuwsen J, Hothuis JJM. Determination of vinca alkaloids in plasma and urine using ion exchange chromatography on silica gel and fluorescence detection. Journal of Chromatography — Biomedical Applications 434: 145–155, 1988b
Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO Journal 1: 945–951, 1982
Welsh MJ, Dedman JR, Brinkley BR. Tubulin and calmodulin. Journal of Cellular Biology 81: 624–634, 1979
Zhou XJ, Boré P, Monjanel S, Zouhir S, Favre R, et al. Pharmacokinetics of navelbine after oral administration in cancer patients. Cancer Chemotherapy and Pharmacology 29: 66–70, 1991
Zhou XJ, Martin M, Placidi M, Cano JP, Rahmani R. In vivo and in vitro pharmacokinetics and metabolism of vinca alkaloids. II. Vinblastine and vincristine. European Journal of Drug Metabolism and Pharmacokinetics 15: 323–332, 1990
Zhou XJ, Zhou-Pan XR, Gauthier T, Lacarelle B, Placidi M, et al. Biotransformation of vindesine by human liver microsomal fractions. Journal de Pharmacie Clinique (Pharmacocinétique: de la recherche à la clinique): 231-235, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhou, XJ., Rahmani, R. Preclinical and Clinical Pharmacology of Vinca Alkaloids. Drugs 44 (Suppl 4), 1–16 (1992). https://doi.org/10.2165/00003495-199200444-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199200444-00002