Summary
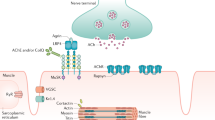
Myasthenia gravis is an autoimmune disorder in which rieuromuscular transmission is impaired by autoantibodies to the acetylcholine receptor (AChR). There are 3 varieties of generalised myasthenia with differing genetic susceptibilities.There is also a purely ocular form in which the weakness is confined to the extraocular muscles, and a neonatal variety which occurs in 20% of babies born to myasthenic mothers due to transplacental passage of the acetylcholine receptor antibody. Another variety of myasthenia occurs several months after treatment with D-penicillamine.
The role of the thymus is suggested by abnormal histology in patients with myasthenia and by the beneficiai effects of thymectomy in more than two-thirds of patients. Thymectomy is indicated in most patients unless the symptoms are minimal or the weakness is confined to the extraocular muscles.
Most patients require treatment with anticholinesterase drugs to prolong the action of acetylcholine at the muscle end-plate. Overdosage of these drugs can provoke a cholinergic weakness.
Remissions can be achieved with corticosteroids in 80% of patients. Immunosuppression with azathioprine is used mainly in patients who do not respond to thymectomy or in those patients who are considered unsuitable for operation. Plasma exchange can cause a rapid but temporary improvement in myasthenia, and has no long term place in its treatment.
Similar content being viewed by others
References
Aquilonius SM, Eckernas SA, Hartvig P, Lindstrom B, Osterman PO. Pharmacokinetics and oral bioavailability of pyridostigmine in man. European Journal of Clinical Pharmacology 48: 423–428,1980
Aquilonius SM, Eckernas SA, Hartvig P, Lindstrom B, Osterman PO. Clinical pharmacology of neostigmine and pyridostigmine in patients with myasthenia gravis. Journal of Neurology, Neurosurgery and Psychiatry 46: 929–935, 1983
Argov Z, Mastalgia FL. Disorders of neuromuscular transmission caused by drugs. New England Journal of Medicine 301: 409–413, 1979
Arsura EL, Bick A, Brunner NG, Namba T, Grob D. High dose intravenous immunoglobulin in the management of myasthenia gravis. Archives of Internal Medicine 146: 1365–1368, 1986
Bell J, Rasserti L, Smoot S. HLA-DQ beta chain polymorphism linked to myasthenia gravis. Lancet 1: 1058–1060, 1986
Bingle JP, Rutherford JD, Woodrow P. Continuous subcutaneous neostigmine in the management of severe myasthenia gravis. British Medical Journal 1: 1050, 1979
Borel JF. Animal experiments with cyclosporin. Triangle 23: 153–158, 1984
Buckingham JM, Howard Jr FM, Bernatz PE, Spencer Payne W, Harrison EG, et al. The value of thymectomy in myasthenia gravis: a computer assisted matched study. Annals of Surgery 184: 453–458, 1976
Calvey TN, Chan K. Plasma pyridostigmine levels in patients with myasthenia gravis. Clinical Pharmacology and Therapeutics 21: 187–193, 1977
Cohen DJ, Loestscher R, Rubin MF, Tilney NL, Carpenter CB, et al. Cyclosporine: a new immunosuppressive agent for organ transplantation. Annals of Internal Medicine 101: 667–682, 1984
Compston DA, Vincent A, Newson-Davis J, Batchelor JR. Clinical pathological HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain 103: 579–601, 1980
Fonseca VF, Havard CWH. Long term treatment of myasthenia gravis with azathioprine. Postgraduate Medical Journal, in press, 1989
Garlep MJ, Dawkins RL, Christiansen FT. HLA antigens and acetylcholine receptor antibodies in penicillamine induced myasthenia gravis. British Medical Journal 286: 338–341, 1983
Havard CWH, Scadding GK. Myasthenia gravis: pathogenesis and current concepts in management. Drugs 26: 174–184, 1983
Kennedy FS, Moersch FP. Myasthenia gravis: a clinical review of 87 cases observed between 1925 and the early part of 1932. Canadian Medical Association Journal 37: 216, 1937
Kornfeld P, Horowitz SH, Genkins G, Papatestas A. Myasthenia gravis unmasked by antiarrhythmic agents. Mt Sinai Journal of Medicine 43: 10–14, 1976
Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis: prevalence, clinical correlates and diagnostic value. Neurology 26: 1054–1059, 1976
Mertens HG, Hertel G, Reuther P, Ricker K. Effect of immunosuppressive drugs (azathioprine). Annals of the New York Academy of Sciences 377: 691–699, 1981
Mier A, Havard CWH. Diaphragmatic myasthenia in mother and child. Postgraduate Medical Journal 61: 725–727, 1985
Murphy J, Murphy SF. Myasthenia gravis in identical twins. Neurology 36: 78–80, 1986
Newsom-Davis J, Wilson SG, Vincent A, Ward CD. Long term effects of repeated plasma exchange in myasthenia gravis. Lancet 1: 464–468, 1979
Noda M, Furutani Y, Takahashi H, Toyosato H, Tanabe T, et al. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding α-subunit precursor of muscle acetylcholine receptor. Nature 305: 818–823, 1983
Osterhuis HJ. The natural history of myasthenia gravis and various procedures influencing its course. In Satoyoshi E (Ed.) Myasthenia gravis: pathogenesis and treatment, pp. 361–371, University of Tokyo Press, Tokyo, 1981
Pascuzzi RM, Coslett HB, Johns TR. Long term corticosteroid treatment of myasthenia gravis: report of 116 patients. Annals of Neurology 15: 291–298, 1984
Pirofsky B. The effect of anti-thymocyte antiserum in progressive myasthenia gravis. Annals of the New York Academy of Sciences 377: 779–785, 1981
Plauché WC. Myasthenia gravis in pregnancy: an update. American Journal of Obstetrics and Gynecology 135(5): 691–697, 1979
Rowland LP. Controversies about the treatment of myasthenia gravis. Journal of Neurology, Neurosurgery and Psychiatry 43: 644–659, 1980
Rowland LP, Hoefer PRA, Aranow HJR, Merritt HH. Fatalities in myasthenia gravis: a review of 39 cases with 26 autopsies. Neurology 6: 307–326, 1956
Tindall RS, Rollins JA, Phillips JJ, Greenlee RG, Wells L, et al. Preliminary results of a double-blind, randomised, placebo-controlled trial of cyclosporine in myasthenia gravis. New England Journal of Medicine 316: 719–724, 1987
White MC, de Silva P, Havard CWH. Plasma pyridostigmine levels in myasthenia gravis. Neurology 31: 145–150, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Havard, C.W.H., Fonseca, V. New Treatment Approaches to Myasthenia Gravis. Drugs 39, 66–73 (1990). https://doi.org/10.2165/00003495-199039010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199039010-00006