Abstract
Background and objective
The individual dosing of drugs that are mainly eliminated unchanged in the urine is made possible by assessing renal function. Most of the methods used are based on serum creatinine (SCr) levels. Cystatin C(CysC) has been proposed as an alternative endogenous marker of the glomerular filtration rate (GFR). Carboplatin is one of the drugs for which elimination is most dependent on the GFR. A prospective clinical trial including 45 patients was conducted to assess the value of serum CysC as a predictor of carboplatin clearance (CL).
Methods
The patients were receiving carboplatin as part of established protocols. Carboplatin was administered as a daily 60-minute infusion at doses ranging from 290 to 1700mg. A population pharmacokinetic analysis was performed using the nonlinear mixed effect modelling NONMEM program according to a two-compartment pharmacokinetic model.
Results
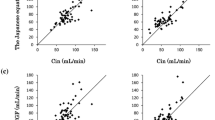
Data from 30 patients were used to test the relationships between carboplatin CL and morphological, biological and demographic covariates previously proposed for prediction of the GFR. The interindividual variability of carboplatin CL decreased from 31% (no covariate) to 14% by taking into account five covariates (SCr, CysC, bodyweight [BW], age and sex). Prospective evaluation of these relationships using the data from the other 15 patients confirmed that the best equation to predict carboplatin CL was based on these five covariates, with a mean absolute percentage error of 13% as an assessment of precision. NONMEM analysis of the whole dataset (n = 45 patients) was performed. The best covariate equation corresponding to the overall analysis was: CL (mL/min) = 110 · (SCr/75)−0.512 · (CysC/1.0)−0.327 · (BW/65)0.474 · (age/ 56)−0.387 · 0.854sex, with SCr in μmol/L, CysC in mg/L, BW in kilograms, age in years and sex = 0 if male and 1 if female. To put the value of CysC as an endogenous marker of the GFR into perspective, covariate equations without SCr were also evaluated; a better prediction was obtained by considering CysC together with age and BW (interindividual variability of 16.6% vs 23.3% for CysC alone).
Conclusion
CysC is a marker of drug elimination that is at least as good as SCr for predicting carboplatin CL. The model based on five covariates was superior to those based on only four covariates (with BW, age and sex combined with either SCr or CysC), indicating that CysC and SCr are not completely redundant to each other. Further pharmacokinetic evaluation is needed to determine whether SCr or CysC is the better marker of renal elimination of other drugs.








Similar content being viewed by others
References
Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130(6): 461–70
Newman DJ. Cystatin Ann Clin Biochem 2002; 39(Pt 2): 89–104
Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate?. Clin Chem 2002; 48(5): 699–707
Dharnidharka VR, Kwon C, Stevens G. Serum cystatin is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002; 40(2): 221–6
Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7(11): 1748–56
Ando M, Minami H, Ando Y, et al. Multi-institutional validation study of carboplatin dosing formula using adjusted serum creatinine level. Clin Cancer Res 2000; 6(12): 4733–8
Chatelut E, Canal P, Brunner V, et al. Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 1995; 87(8): 573–80
Shen M, Schilder RJ, Obasaju et al. Population pharmacokinetic and limited sampling models for carboplatin administered in high-dose combination regimens with peripheral blood stem cell support. Cancer Chemother Pharmacol 2002; 50(3): 243–50
Chatelut E, Pivot X, Otto J, et al. A limited sampling strategy for determining carboplatin AUC and monitoring drug dosage. Eur J Cancer 2000; 36(2): 264–9
LeRoy AF, Wehling ML, Sponseller HL, et al. Analysis of platinum in biological materials by flameless atomic absorption spectrophotometry. Biochem Med 1977; 18(2): 184–91
Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng 1982; 8(3): 195–222
Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin levels other than renal function and the impact on renal function measurement. Kidney Int 2004; 65(4): 1416–21
Galteau MM, Guyon M, Gueguen R, et al. Determination of serum cystatin C: biological variation and reference values. Clin Chem Lab Med 2001; 39(9): 850–7
Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 2000; 37(Pt 1): 49–59
Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 1995; 47(1): 312–8
Norlund L, Fex G, Lanke J, et al. Reference intervals for the glomerular filtration rate and cell-proliferation markers: serum cystatin C and serum beta 2-microglobulin/cystatin C-ratio. Scand J Clin Lab Invest 1997; 57(6): 463–70
Pergande M, Jung K. Sandwich enzyme immunoassay of cysta-tin C in serum with commercially available antibodies. Clin Chem 1993; 39(9): 1885–90
O’Riordan S, Ouldred E, Brice S, et al. Serum cystatin C is not a better marker of creatinine or digoxin clearance than serum creatinine. Br J Clin Pharmacol 2002; 53(4): 398–402
Seronie-Vivien S, Galteau MM, Carlier MC, et al. Improving the interlaboratory variation for creatinine serum assay [in French]. Ann Biol Clin (Paris) 2004; 62(2): 165–75
Léger F, Seronie-Vivien S, Makdessi J, et al. Impact of the biochemical assay for serum creatinine measurement on the individual carboplatin dosing: a prospective study. Eur J Cancer 2002; 38(1): 52–6
Finney H, Newman DJ, Gruber W, et al. Initial evaluation of cystatin C measurement by particle-enhanced immu-nonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 1997; 43(6 Pt 1): 1016–22
Lemann J, Bidani AK, Bain RP, et al. Use of the serum creatinine to estimate glomerular filtration rate in health and early diabetic nephropathy. Collaborative Study Group of Angiotensin Converting Enzyme Inhibition in Diabetic Nephropathy. Am J Kidney Dis 1990; 16(3): 236–43
Shemesh O, Golbetz H, Kriss JP, et al. Limitations of creatinine as a filtration marker in glomerulopathy patients. Kidney Int 1985; 28(5): 830–8
Wasen E, Isoaho R, Mattila K, et al. Serum cystatin C in the aged: relationships with health status. Am J Kidney Dis 2003; 42(1): 36–43
Bokenkamp A, van Wijk JA, Lentze MJ, et al. Effect of corticosteroid therapy on serum cystatin C and β2-microglobulin concentrations. Clin Chem 2002; 48(7): 1123–6
Acknowledgements
The authors thank Dade Behring, Marburg, Germany, for providing the analyser and kits for the measurement of cystatin C. None of the authors have any conflicts of interest to declare with respect to the contents of this study.
The study was funded by the ‘Conseil Scientifique de l’Institut Claudius-Regaud’ and the ‘Ligues Départementales de Lutte Contre le Cancer de la Région Midi-Pyrénées’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, F., Séronie-Vivien, S., Gladieff, L. et al. Cystatin C as a New Covariate to Predict Renal Elimination of Drugs. Clin Pharmacokinet 44, 1305–1316 (2005). https://doi.org/10.2165/00003088-200544120-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200544120-00009