Abstract
Goserelin is a synthetic decapeptide analogue of luteinising hormone-releasing hormone (LHRH). For experimental purposes it has been administered subcutaneously as an aqueous solution, but for therapeutic use it is formulated as subcutaneous depots releasing goserelin over periods of 1 (3.6mg) or 3 (10.8mg) months.
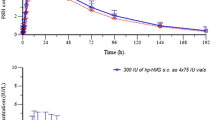
Pharmacokinetic data have been generated using a specific radioimmunoassay. When administered as a solution, goserelin is rapidly absorbed and eliminated from serum with a mean elimination half-life (t1/2β) of 4.2 hours in males and 2.3 hours in females. The shapes of the observed serum goserelin profiles following administration of the depots are primarily determined by the rate of goserelin release from the biodegradable lactide-glycolide copolymer matrix over periods of 1 or 3 months. There is no clinically relevant accumulation of goserelin during multiple administration of these depots.
Goserelin is extensively metabolised prior to excretion. Its pharmacokinetics are unaffected by hepatic impairment, but the mean t1/2β increases to 12.1 hours in patients with severe renal impairment. This suggests that the total renal clearance (renal metabolism and unchanged drug) is decreased in patients with renal dysfunction. It is unnecessary to adjust the dose or administration interval when the depot formulations are administered to elderly patients or to those with impaired renal or hepatic function.
Administration of a goserelin 3.6mg or 10.8mg depot results in an initial increase of luteinising hormone (LH) levels and in increases of serum testosterone or oestradiol levels in males and females, respectively. This is followed by a decrease in serum LH levels and suppression of testosterone or oestradiol to within the castrate or menopausal range, respectively. Subsequently, throughout treatment with goserelin depots, serum testosterone or oestradiol levels remain suppressed.
Clinical outcomes following treatment of patients with prostate cancer, breast cancer and benign gynaecological conditions with goserelin are described briefly.

















Similar content being viewed by others
Notes
1 Zoladex is a trade mark, the property of the AstraZeneca group of companies.
References
Goldspiel BR, Kohler DR. Goserelin acetate implant: a depot luteinizing hormone-releasing hormone analog for advanced prostate cancer. Ann Pharmacother 1991; 25: 796–804.
Chrisp P, Goa KL. Goserelin: a review of its pharmacodynamic and pharmacokinetic properties, and clinical use in sex hormone related conditions. Drugs 1991; 41: 254–88.
Brogden RN, Faulds D. Goserelin: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in prostate cancer. Drugs Aging 1995; 6: 324–43.
Perry CM, Brogden RN. Goserelin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in benign gynaecological disorders. Drugs 1996; 51: 319–46.
Ahmann FR, Citrin DL, deHaan HA, et al. ‘Zoladex’: a sustained-release, monthly leuteinizing hormone-releasing hormone analogue for the treatment of advanced prostate cancer. J Clin Oncol 1987; 5: 912–7.
Kotake T, Usami M, Sonoda T, et al. LH-RH agonist, ‘Zoladex’ (goserelin), depot formulation in the treatment of prostatic cancer: randomised dose-finding trial in Japan. Am J Clin Oncol Cancer Clin Trials 1988; 11: S108–11.
Hutchinson FG, Furr BJA. Biodegradable polymer systems for the sustained release of polypeptides. J Control Release 1990; 13: 279–94.
Dijkman GA, Fernandez del Moral P, Piasman JWMH, et al. A new longer-acting LHRH analogue depot: preliminary results of a Dutch open phase II clinical study on a 10.8mg ‘Zoladex’ 3-monthly depot. Eur Urol 1990; 18 Suppl. 3: 22–5.
Moore RH, Barker Y, Porter ET. Radioimmunoassay of ICI 118,630 release in-vivo from ‘Zoladex’ depots [abstract]. Pharm Weekblad Sci Ed 1988; 10: 57.
Clayton RN, Bailey LC, Cottam J, et al. A radioimmunoassay for GnRH agonist analogue in serum of patients with prostate cancer treated with D-Ser (tBu)6 Aza Gly10 GnRH. Clin Endocrinol 1985; 22: 453–62.
Perren TJ, Clayton RN, Blackledge G, et al. Pharmacokinetic and endocrinological parameters of a slow-release depot preparation of the GnRH analogue ICI 118630 (‘Zoladex’) compared with a subcutaneous bolus and continuous subcutaneous infusion of the same drug in patients with prostatic cancer. Cancer Chemother Pharmacol 1986; 18: 39–43.
Adam HK. The importance of the pharmacokinetics of hormonal drugs in the therapy of prostatic carcinoma [abstract]. Acta Urol Ital 1992; 6 Suppl. 2: 9–17.
Barnfield DJ, Barker Y, Knott S, et al. The disposition of ‘Zoladex’™ (ICI 118,630) in laboratory animals [abstract]. Pharm Weekblad Sci Ed 1988; 10: 56.
AstraZeneca. Study 0021, 1985 (data on file).
AstraZeneca. Study 2506, 1988 (data on file).
Adam HK, Barker Y, Hutchinson FG, et al. ‘Zoladex’: a one month duration LH-RH agonist [abstract]. Pharm Weekblad Sci Ed 1988; 10:57.
Barron JL, Millar RP, Searle D. Metabolic clearance and plasma half-disappearance time of D-TRP6 and exogenous luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 1982; 54: 1169–73.
AstraZeneca. Study 1702P, 1986 (data on file).
AstraZeneca. Study 0017, 1986 (data on file).
AstraZeneca. Study 0016, 1985 (data on file).
AstraZeneca. Study 2607, 1989 (data on file).
AstraZeneca. Study 2114, 1990 (data on file).
AstraZeneca. Study 7822-2C, 1990 (data on file).
Dijkman GA, Debruyne FMJ, Fernandez del Moral P, et al. A randomised trial comparing the safety and efficacy of ‘Zoladex’ 10.8-mg depot, administered every 12 weeks, to that of the ‘Zoladex’ 3.6-mg depot, administered every 4 weeks, in patients with advanced prostate cancer. Eur Urol 1995; 27: 43–6.
AstraZeneca. Study HQ0001, 1994 (data on file).
AstraZeneca. Study 1805, 1994 (data on file).
Fernandez del Moral P, Dijkman GA, Debruyne FMJ, et al. Three-month depot of goserelin acetate: clinical efficacy and endocrine profile. Urology 1996; 48: 894–900.
AstraZeneca. Study IL0024, 1999 (data on file).
AstraZeneca. Study HQ0002, 1994 (data on file).
AstraZeneca. Study 1804, 1994 (data on file).
AstraZeneca. Study G3, 1987 (data on file).
AstraZeneca. Study G4, 1988 (data on file).
AstraZeneca. Study 0024, 1989 (data on file).
AstraZeneca. Study 0031, 1990 (data on file).
Barnfield DJ, Warrander A. The metabolism of ‘Zoladex’™ (ICI 118,630) by laboratory animals [abstract]. Pharm Weekblad Sci Ed 1988; 10: 56.
Allen JM, O’shea JP, Mashiter K, et al. Advanced carcinoma of the prostate: treatment with a gonadotrophin releasing hormone agonist. BMJ 1983; 286: 1607–9.
Murray RML, Pitt P, Lawson P, et al. Treatment of advanced prostatic cancer with an LHRH analogue [abstract]. Br J Urol 1987; 59: 182.
Thomas EJ, Jenkins J, Lenton EA, et al. Endocrine effects of goserelin, a new depot luteinising hormone releasing hormone agonist. BMJ 1986; 293: 1407–8.
Dowsett M, Cantwell B, Lal A, et al. Suppression of postmenopausal ovarian steroidogenesis with the luteinizing hormone-releasing hormone agonist goserelin. J Clin Endocrinol Met 1988; 66: 672–7.
Blarney RW, Jonat W, Kaufmann M, et al. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer 1992; 28A: 810–4.
Sarosdy MF, Schellhammer PF, Soloway MS, et al. Endocrine effects, efficacy and tolerability of a 10.8-mg depot formulation of goserelin acetate administered every 13 weeks to patients with advanced prostate cancer. BJU Int 1999; 83: 801–6.
Hall MC, Fritzsch RJ, Sagalowsky AI, et al. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology 1999; 53: 898–902.
AstraZeneca. Study 1803, 1994 (data on file).
Usami M, Kotake T, Matsuda M, et al. Clinical efficacy of longterm treatment with LH-RH analogue, ICI 118630 (Zoladex), in prostatic cancer patients: the Zoladex Multicenter Study Group. Hinyokika Kiyo 1988; 34: 2059–66.
Kaisary AV, Tyrrell CJ, Peeling WB, et al. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 1991; 67: 502–8.
Vogelzang NJ, Chodak GW, Soloway MS, et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomised trial. Urology 1995; 46: 220–6.
Soloway MS, Cassileth BR, Vogelzang NJ, et al. Patient’s choice of treatment in stage D prostate cancer. In: Recent advances in urological cancer diagnosis and treatment. Paris: Scientific Communications International, 1990: 72–4.
Cassileth BR, Soloway MS, Vogelzang NJ, et al. Quality of life and psychosocial status in stage D prostate cancer. Qual Life Res 1992; 1:323–9.
Thorpe SC, Azmatullah S, Fellows GJ, et al. A prospective, randomised study to compare goserelin acetate (Zoladex) vs Cyprostat (cyproterone acetate) vs a combination of the two in the treatment of metastatic prostatic carcinoma. Eur Urol 1996; 29: 47–54.
Waymont B, Lynch TH, Dunn JA, et al. Phase III randomised study of Zoladex versus stilboestrol in the treatment of advanced prostate cancer. Br J Urol 1992; 69: 614–20.
Citrin DL, Resnick MI, Guinan P, et al. A comparison of Zoladex and DES in the treatment of advanced prostate cancer: results of a randomised multicenter trial. Prostate 1991; 18: 139–46.
Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997; 337: 295–300.
Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive cancer. N Engl J Med 1999; 341: 1781–8.
Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Lancet 1995; 346: 265–9.
Caubet JF, Tosteson TD, Dong EW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomised controlled trials using nonsteroidal antiandrogens. Urology 1997; 49: 71–8.
Klotz LH, Newman T. Does maximal androgen blockade (MAB) improve survival? A critical appraisal of the evidence. Can J Urol 1996; 3: 246–50.
Bennett CL, Tosteson TD, Schmitt B, et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomised controlled trials and 4128 patients using flutamide. Prost Cancer Prost Dis 1999; 2: 4–8.
Denis LJ, Keuppens F, Smith PH, et al. Maximal androgen blockage: final analysis of EORTC Phase III trial 30853. Eur Urol 1998; 33: 144–51.
Iversen P, Rasmussen F, Klarskov P, et al. Long-term results of Danish Prostatic Cancer Group Trial 86. Cancer 1993; 72 Suppl.: 3851–4.
Tyrrell CJ. An international, multi-centre, randomised trial comparing ‘Zoladex’ (goserelin) to maximal androgen blockade (MAB) in advanced prostate cancer: final survival results [abstract]. Eur Urol 1998; 33 Suppl. 1: 46.
Pilepich MV, Krall JM, Al-Sarraf M, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomised comparative trial of Radiation Therapy Oncology Group. Urology 1995; 45: 616–23.
Montironi R, Diamanti L, Santinelli A, et al. Effect of total androgen ablation on pathological stage and resection limit status of prostate cancer: initial results of the Italian PROSIT study. Pathol Res Pract 1999; 195: 201–8.
Debruyne FM, Dijkman GA, Lee DCH, et al. A new long acting formulation of the luteinizing hormone-releasing hormone analogue, goserelin: results of studies in prostate cancer. J Urol 1996; 155: 1352–4.
Blarney RW, Jonat W, Kaufmann M, et al. Survival data relating to the use of goserelin depot in the treatment of premenopausal advanced breast cancer [letter]. Eur J Cancer 1993; 29A: 1498.
Williams MR, Walker KJ, Blarney RW, et al. The use of an LH-RH agonist (ICI 118630, Zoladex) in advanced premenopausal breast cancer. Br J Cancer 1986; 53: 629–36.
Brambilla C, Escobedo A, Artioli R, et al. Treatment of premenopausal advanced breast cancer with goserelin: a longacting luteinizing hormone releasing hormone agonist. Anticancer Drugs 1992; 3: 3–8.
Bajetta E, Zilembo N, Buzzoni R, et al. Goserelin in premenopausal advanced breast cancer: clinical and endocrine evaluation of responsive patients. Oncology 1994; 51: 262–9.
Taylor CW, Green S, Dalton WS, et al. Multicenter randomised clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 1998; 16: 994–9.
Buzonni R, Biganzoli L, Bajetta E, et al. Combination goserelin and tamoxifen therapy in premenopausal advanced breast cancer: a multicentre study by the ITMO group: Italian trials in medical oncology. Br J Cancer 1995; 71: 111–4.
Jonat W, Kaufmann M, Blarney RW, et al. A randomised study to compare the effect of the luteinising hormone releasing hormone (LHRH) analogue goserelin with or without tamoxifen in pre- and perimenopausal patients with advanced breast cancer. Eur J Cancer 1995;31A: 137–42.
Klijn JGM, Blarney RW, Boccardo F, et al. A new standard treatment for advanced premenopausal breast cancer: a metaanalysis of the Combined Hormonal Agent Trialists’ Group (CHAT) [abstract 405]. Eur J Cancer 1998; 34 Suppl. 5: S90.
Klijn JGM, Blarney RW, Boccardo F, et al. Combination LHRH-agonist plus tamoxifen treatment is superior to medical castration alone in premenopausal metastatic breast cancer: a sub-group and meta-analysis of the Combined Hormonal Agent Trialists’ Group (CHAT) [abstract 4]. Breast Cancer Res Treat 1998; 50: 227.
Baum M. Adjuvant treatment of premenopausal breast cancer with ‘Zoladex’ and tamoxifen: results from the ZIPP trial organised by the Cancer Research Campaign (CRC) Breast Cancer Trials Group, the Stockholm Breast Cancer Study Group, the South East Sweden Breast Cancer Group and Gruppo Interdisciplinare Valutazione Interventi in Oncologia (GIVIO) [abstract 24]. Breast Cancer Res Treat 1999; 57: 30.
Jakesz R, Gnant M, Hausmaninger H, et al. Combination goserelin and tamoxifen is more effective than CMF in premenopausal patients with hormone-responsive tumors in a multicenter trial of the Austrian Breast Cancer Study Group (ABCSG) [abstract 2]. Breast Cancer Res Treat 1999; 57: 25.
Boccardo F, Rubagotti A, Amoroso D, et al. CMF vs. tamoxifen (TAM) plus ovarian suppression (OS) as adjuvant treatment of ER positive (ER+) pre-perimenopausal breast cancer (BCA) patients [abstract 072]. Breast 1999; 8: 233.
Davidson N, O’Neill A, Vukov A, et al. Effect of chemohormonal therapy in premenopausal, node-positive, receptor-positive breast cancer: an Eastern Co-operative Oncology Group Phase III Intergroup Trial (E5188, INT-0101) [abstract 069]. Breast 1999; 8: 233.
Kuter I. Breast cancer highlights [ASCO 1999 Critical Commentaries]. Oncologist 1999; 4: 299–308.
Jonat W, ZEBRA Study Group. The ‘ZEBRA’ study: ‘Zoladex’ (goserelin) vs CMF as adjuvant therapy in the management of node positive stage II breast cancer in pre/peri-menopausal women aged 50 years or less [abstract 330]. Breast Cancer Res Treat 1998; 50: 283.
Shaw RW, on behalf of the Study Group. An open randomized comparative study of the effect of goserelin depot and danazol in the treatment of endometriosis. Fertil Steril 1992; 58: 265–72.
Rock JA, Truglia JA, Caplan RJ, et al. ‘Zoladex’ (goserelin acetate implant) in the treatment of endometriosis: a randomized comparison with danazol. Obstet Gynecol 1993; 82: 198–205.
Reichel RP, Schweppe KW, on behalf of the Study Group. Goserelin (‘Zoladex’) depot in the treatment of endometriosis. Fertil Steril 1992; 57: 1197–202.
Howell R, Crook D, Edmonds DK, et al. Gonadotropin-releasing hormone analogue (goserelin) plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril 1995; 64: 474–81.
Moghissi KS, Schlaff WD, Olive DL, et al. Goserelin acetate (‘Zoladex’) with or without hormone replacement therapy for the treatment of endometriosis. Fertil Steril 1998; 69: 1056–62.
Kiesel L, Schweppe KW, Sillem M, et al. Should add-back therapy for endometriosis be deferred for optimal results? Br J Obstet Gynaecol 1996; 103: 15–7.
Kiilholma P, Korhonen M, Tuimala R, et al. Comparison of the gonadotropin-releasing hormone agonist goserelin acetate alone versus goserelin combined with estrogen-progestogen add-back therapy in the treatment of endometriosis. Fertil Steril 1995; 64: 903–8.
Venturini PL, Anserini P, Fasce V, et al. Treatment of endometriosis with goserelin depot, a long-acting gonadotropin-releasing hormone agonist analog: endocrine and clinical results. Fertil Steril 1990; 54: 1021–7.
Schweppe KW, Steinmueller H, Mettler L. ‘Zoladex’ in the treatment of endometriosis: German experiences. Adv Reprod Endocrinol 1990; 1: 179–91.
AstraZeneca, Study IL0026, 1998 (Data on file).
AstraZeneca, Study IL0027, 2000 (Data on file).
Maekaeraeinen L, Roennberg L, Kauppila A. Medroxyprogesterone acetate supplementation diminishes the hypoestrogenic side effects of gonadotropin-releasing hormone agonist without changing its efficacy in endometriosis. Fertil Steril 1996; 65: 29–34.
Stovall TG, Jenison EL, on behalf of the Study Group. A comparative study of adjuvant GnRH agonist (‘Zoladex’) therapy vs immediate surgery in the treatment of uterine myoma [abstract 1734]. 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sep 15–20; Singapore. Int J Gynaecol Obstet 1991; Spec Iss (Bk 3): 308.
Gerris J, Degueldre M, Peters AAW, et al. The place of ‘Zoladex’ in deferred surgery for uterine fibroids. Horm Res 1996; 45: 279–84.
Audebert AJM, Madenelat P, Querleu D, et al. Deferred versus immediate surgery for uterine fibroids: clinical trial results. Br J Obstet Gynaecol 1994; 101 Suppl. 10: 29–32.
Candiani GB, Vercellini P, Fedele L, et al. Use of goserelin depot, a gonadotrophin-releasing hormone agonist, for the treatment of menorrhagia and severe anemia in women with leiomyomata uteri. Acta Obstet Gynecol Scand 1990; 69: 413–5.
Stampe-Soerensen S, Colov NP, Vejerslev LO. Pre- and postoperative therapy with GNRH agonist in preparation for endometrial resection [abstract 063]. Gynaecol Endoscop 1995; 4 Suppl. 1: 35.
Garry R, Khair A, Mooney P, et al. A comparison of goserelin and danazol as endometrial thinning agents prior to endometrial laser ablation. Br J Obstet Gynaecol 1996; 103: 339–44.
Goserelin acetate. In: Dollery C, editor. Therapeutic drugs. London: Churchill Livingstone, 1999: G82–6.
Zoladex investigators’ brochure. 8th ed. AstraZeneca, 2000.
Hämmerlein A, Derendorf H, Lowenthal DT. Pharmacokinetic and pharmacodynamic changes in the elderly: clinical implications. Clin Pharmacokinet 1998; 35: 49–64.
Tsujimoto G, Hashimoto K, Hiffman BB. Pharmacokinetic and pharmacodynamic principles of drug therapy in old age: pt 1. Int J Clin Pharmacol 1989; 27: 13–26.
O’Mahony MS, Woodhouse KW. Age, environmental factors and drug metabolism. Pharmacol Ther 1994; 61: 279–87.
Acknowledgements
The author would like to thank the many colleagues and clinical trialists for their contributions to the AstraZeneca data on file which have been included in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cockshott, I.D. Clinical Pharmacokinetics of Goserelin. Clin Pharmacokinet 39, 27–48 (2000). https://doi.org/10.2165/00003088-200039010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200039010-00003