Abstract
Background and objective
Elderly patients may be at higher risk of drug-drug interactions (DDIs) because of polypharmacy. This study evaluated age-specific differences in the prevalence of clinically relevant potential DDIs (pDDIs) in ambulatory dyslipidaemic patients treated with an HMG-CoA reductase inhibitor (statin). We hypothesised that elderly patients are at higher risk for pDDIs because of the presence of more drugs and drugs with a higher potential for DDIs in this age group.
Methods
A total of 2742 dyslipidaemic ambulatory patients treated with a statin were included in this cross-sectional study. Drug treatment was screened for clinically relevant pDDIs using an electronic drug interaction program (DRUG-REAX® System).
Results
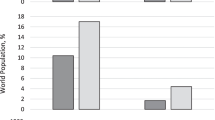
The study sample consisted of 483 (17.6%) patients aged ≤54 years, 732 (26.7%) aged 55–64 years, 924 (33.7%) aged 65–74 years and 603 (22.0%) patients aged ≥75 years. Patients ≥75 years had significantly more pharmacologically active substances prescribed than patients aged ≤54 years (mean 5.8 vs 3.8, respectively; p < 0.001). Cardiovascular diseases such as coronary heart disease, heart failure or arrhythmias were also significantly more prevalent in patients aged ≥75 years than in younger patients. The overall prevalence of pDDIs increased significantly from 7.9% in those aged ≤54 years to 18.4% in patients aged ≥75 years (p < 0.001). The frequency of both pDDIs associated with statins and non-statin pDDIs increased with age. Risk factors for pDDIs in patients aged ≥75 years were arrhythmias, heart failure and the number of pharmacologically active substances prescribed. The more frequent prescription of cardiovascular drugs with a high potential for pDDIs (e.g. amiodarone and digoxin) in patients aged ≥75 years was mainly responsible for the observed increases in statin and non-statin pDDIs in this age group.
Conclusions
Compared with younger patients, elderly dyslipidaemic patients are at a higher risk for clinically relevant pDDIs, mainly because of a higher number of drugs prescribed. In addition, patients aged ≥75 years were prescribed more drugs with a high potential for DDIs, especially drugs used for the treatment of arrhythmias and heart failure. The risk for adverse reactions associated with pDDIs may often be reduced by dose adjustment, close monitoring or selection of an alternative drug.






Similar content being viewed by others
References
Doucet J, Chassagne P, Trivalle C, et al. Drug-drug interactions related to hospital admissions in older adults: a prospective study of 1000 patients. J Am Geriatr Soc 1996 Aug; 44(8): 944–8
Kohler GI, Bode-Boger SM, Busse R, et al. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther 2000 Nov; 38(11): 504–13
Straubhaar B, Krähenbühl S, Schlienger RG. The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug Saf 2006; 29(1): 79–90
Egger SS, Drewe J, Schlienger RG. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol 2003 Mar; 58(11): 773–8
Chen YF, Avery AJ, Neil KE, et al. Incidence and possible causes of prescribing potentially hazardous/contraindicated drug combinations in general practice. Drug Saf 2005; 28(1): 67–80
Herrlinger C, Klotz U. Drug metabolism and drug interactions in the elderly. Best Pract Res Clin Gastroenterol 2001 Dec; 15(6): 897–918
Jankel CA, Fitterman LK. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf 1993 Jul; 9(1): 51–9
Egger T, Dormann H, Ahne G, et al. Identification of adverse drug reactions in geriatric inpatients using a computerised drug database. Drugs Aging 2003; 20(10): 769–76
Seymour RM, Routledge PA. Important drug-drug interactions in the elderly. Drugs Aging 1998 Jun; 12(6): 485–94
Bjerrum L, Andersen M, Petersen G, et al. Exposure to potential drug interactions in primary health care. Scand J Prim Health Care 2003 Sep; 21(3): 153–8
Rätz Bravo AE, Tchambaz L, Krähenbühl-Melcher A, et al. Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf 2005; 28(3): 263–75
Rätz Bravo AE, Tchambaz L, Krähenbühl-Melcher A, et al. Prevalence of potential drug-drug interactions in ambulatory patients with statin therapy. Schweiz Rundsch Med Prax 2006 Feb 1; 95(5): 139–46
WHO drug dictionary. Version 01–3, third quarter. Uppsala: WHO Collaborating Centre, 2001
Thomson Micromedex. DRUG-REAX® System [online]. Available from URL: http://www.thomsonhc.com/home/dispatch [Accessed 2002 Apr 30]
ICH Topic E2A. Clinical safety data management: definitions and standards for expedited reporting. London: The European Agency for the Evaluation of Medicinal Products, 1994
Stockley IH, editor. Drug interactions. London: The Pharmaceutical Press, 1999
Tatro DS, editor. Drug interaction facts™. Updated 2003 Jan. St Louis (MO): Facts and Comparisons, 2003
e-mediat. Pharmavista databases: interactions [online]. Available from URL: http://www.pharmavista.ch [Accessed 2002 Apr 30]
Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist 1979; 6: 65–70
Sauro J, Lewis JR. Estimating completion rates from small samples using binomial confidence intervals: comparisons and recommendations. In: Proceedings of the Human Factors and Ergonomics society: 49th Annual Meeting; 2005 Sep 26–30; Orlando (FL). Santa Monica (CA): Human Factors and Ergonomics Society, 2005: 2100–4
Roten L, Schoenenberger RA, Krähenbühl S, et al. Rhabdomyolysis in association with simvastatin and amiodarone. Ann Pharmacother 2004 Jun; 38(6): 978–81
Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet 2002; 41(5): 343–70
Pasternak RC, Smith Jr SC, Bairey-Merz CN, et al. ACC/AHA/ NHLBI clinical advisory on the use and safety of statins. Stroke 2002 Sep; 33(9): 2337–41
Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001 May 16; 285(19): 2486–97
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I. Aging arteries: a “set up” for vascular disease. Circulation 2003 Jan 7; 107(1): 139–46
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999 Sep 2; 341(10): 709–17
Shah KB, Rao K, Sawyer R, et al. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol 2005 Sep 6; 46(5): 845–9
Wrenger E, Müller R, Moesenthin M, et al. Interaction of spironolactone with ACE inhibitors or angiotensin receptor blockers: analysis of 44 cases. BMJ 2003 Jul 19; 327(7407): 147–9
Gheorghiade M, Adams Jr KF, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation 2004 Jun 22; 109(24): 2959–64
Miura T, Kojima R, Sugiura Y, et al. Effect of aging on the incidence of digoxin toxicity. Ann Pharmacother 2000 Apr; 34(4): 427–32
Marik PE, Fromm L. A case series of hospitalized patients with elevated digoxin levels. Am J Med 1998 Aug; 105(2): 110–5
Steiness E. Diuretics, digitalis and arrhythmias. Acta Med Scand Suppl 1981; 647: 75–8
Wakasugi H, Yano I, Ito T, et al. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin Pharmacol Ther 1998 Jul; 64(1): 123–8
Bachmakov I, Werner U, Endress B, et al. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol 2006 Jun; 20(3): 273–82
Wermeling DP, Field CJ, Smith DA, et al. Effects of long-term oral carvedilol on the steady-state pharmacokinetics of oral digoxin in patients with mild to moderate hypertension. Pharmacotherapy 1994 Sep–Oct; 14(5): 600–6
De Mey C, Brendel E, Enterling D. Carvedilol increases the systemic bioavailability of oral digoxin. Br J Clin Pharmacol 1990 Apr; 29(4): 486–90
Sakaeda T, Fujino H, Komoto C, et al. Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res 2006 Mar; 23(3): 506–12
Triscari J, Swanson BN, Willard DA, et al. Steady state serum concentrations of pravastatin and digoxin when given in combination. Br J Clin Pharmacol 1993 Sep; 36(3): 263–5
Garnett WR, Venitz J, Wilkens RC, et al. Pharmacokinetic effects of fluvastatin in patients chronically receiving digoxin. Am J Med 1994 Jun 6; 96(6A): 84S–6S
Lemma GL, Wang Z, Hamman MA, et al. The effect of short-and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther 2006 Mar; 79(3): 218–30
Sutton D, Butler AM, Nadin L, et al. Role of CYP3A4 in human hepatic diltiazem N-demethylation: inhibition of CYP3A4 activity by oxidized diltiazem metabolites. J Pharmacol Exp Ther 1997 Jul; 282(1): 294–300
Ohyama K, Nakajima M, Suzuki M, et al. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol 2000 Mar; 49(3): 244–53
Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004 Jun 15; 109 (23 Suppl. 1): III50–7
Martin J, Krum H. Cytochrome P450 drag interactions within the HMG-CoA reductase inhibitor class: are they clinically relevant? Drug Saf 2003; 26(1): 13–21
Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf 2000 Jun; 22(6): 441–57
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006 Apr 17; 97(8A): 52C–60C
Snowdon J. Is depression more prevalent in old age? Aust N Z J Psychiatry 2001 Dec; 35(6): 782–7
Stewart DE. Physical symptoms of depression: unmet needs in special populations. J Clin Psychiatry 2003; 64Suppl. 7: 12–6
Nelson JC. Diagnosing and treating depression in the elderly. J Clin Psychiatry 2001; 62Suppl. 24: 18–22
Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003 Dec 8–22; 163(22): 2716–24
Acknowledgements
This study was financially supported by Bristol-Myers Squibb, GmbH, Baar, Switzerland. The sponsors had no influence on the study design, analysis and interpretation of data or writing of the manuscript.
Stephan Krähenbühl is supported by a grant from the Swiss National Science Foundation (310’000-112483/1). Stephan Krähenbühl and Lorenzo Hess have received grants from Bristol-Myers Squibb, GmbH, Baar, Switzerland. Raymond Schlienger is employed by Novartis Pharma, Switzerland. None of the authors has any conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egger, S.S., Bravo, A.E.R., Hess, L. et al. Age-Related Differences in the Prevalence of Potential Drug-Drug Interactions in Ambulatory Dyslipidaemic Patients Treated with Statins. Drugs Aging 24, 429–440 (2007). https://doi.org/10.2165/00002512-200724050-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200724050-00006