Abstract
Verteporfin (Visudyne®) therapy (photodynamic therapy with intravenous liposomal verteporfin) is the first treatment to effectively prevent the loss of visual acuity in patients with subfoveal choroidal neovascularisation (CNV) secondary to age-related macular degeneration (AMD), pathological myopia or presumed ocular histoplasmosis syndrome (POHS).
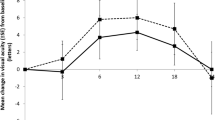
In adult patients with classic subfoveal CNV or occult with no classic subfoveal CNV secondary to AMD, or subfoveal CNV secondary to pathological myopia or POHS, verteporfin therapy slows or prevents loss of visual acuity. In well designed clinical trials, verteporfin therapy was superior to placebo in patients with subfoveal classic-containing CNV and occult with no classic CNV secondary to AMD at 12 and/or 24 months (Treatment of Age-related macular degeneration with Photodynamic therapy [TAP] Investigation and Verteporfin In Photodynamic therapy [VIP-AMD] trial) and in patients with pathological myopia at 12 months (Verteporfin In Photodynamic therapy [VIP-PM] trial). Limited data suggest that verteporfin therapy also prevents loss of visual acuity in patients with subfoveal CNV secondary to POHS.
Verteporfin therapy was generally well tolerated in clinical trials; most adverse events were mild to moderate in intensity and transient. The most frequently reported verteporfin therapy-related adverse events (incidence >2%) were visual disturbance, injection-site reactions, photosensitivity reactions and infusion-related back pain. Approximately 5% of patients with occult with no classic subfoveal CNV secondary to AMD reported severe vision decrease within 7 days of treatment in clinical trials; 3 months later, several patients had recovered some of this loss.
Conclusion: Photodynamic therapy with verteporfin, the first photosensitiser approved for the treatment of subfoveal CNV, is a well tolerated treatment that stabilises or slows visual acuity loss in adult patients with predominantly classic or occult with no classic subfoveal CNV secondary to AMD, and subfoveal CNV secondary to pathological myopia or POHS. Thus, verteporfin therapy provides a valuable option for the management of these patients for whom treatment options are few, and should be considered as a first-line therapy in these difficult-to-manage conditions.
Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
Richter AM, Waterfield E, Jain AK, et al. In vitro evaluation of phototoxic properties of four structurally related benzoporphyrin derivatives. Photochem Photobiol 1990; 52: 495–500
Aveline B, Hasan T, Redmond RW. Photophysical and photosensitizing properties of benzoporphyrin derivative monoacid ring A (BPD-MA). Photochem Photobiol 1994 Mar; 59(3): 328–35
Richter AM, Waterfield E, Jain AK, et al. Photosensitising potency of structural analogues of benzoporphyrin derivative (BPD) in a mouse tumour model. Br J Cancer 1991; 63: 87–93
Schmidt-Erfurth U, Bauman MD, Gragoudas E, et al. Photodynamic therapy of experimental choroidal melanoma using lipo-protein-delivered benzoporphyrin. Ophthalmology 1994; 101: 89–99
Pooler JP, Valenzeno DP. Dye-sensitized photodynamic inactivation of cells. Med Phys 1981; 8(5): 614–28
Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol 2000; 45(3): 195–214
Allison BA, Pritchard PH, Levy JG. Evidence for low-density lipoprotein receptor-mediated uptake of benzoporphyrin derivative [published erratum appears in Br J Cancer 1995 Jan; 71: 214]. Br J Cancer 1994; 69: 833–9
Schmidt-Erfurth U, Hasan T, Schomacker K, et al. In vivo uptake of liposomal benzoporphyrin derivative and photothrombosis in experimental corneal neovascularization. Lasers Surg Med 1995; 17: 178–88
Richter AM, Kelly B, Chow J, et al. Preliminary studies on a more effective phototoxic agent than hematoporphyrin. J Natl Cancer Inst 1987; 79: 1327–32
Rousset N, Vonarx V, Eléouet S, et al. Cellular distribution and phototoxicity of benzoporphyrin derivative and Photofrin. Res Exp Med (Berl) 2000 Jun; 199(6): 341–57
Michels S, Schmidt-Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci 2003 May; 44(5): 2147–54
Schnurrbusch UE, Welt K, Horn L-C, et al. Histological findings of surgically excised choroidal neovascular membranes after photodynamic therapy. Br J Ophthalmol 2001 Sep; 85(9): 1086–91
Ghazi NG, Jabbour NM, De La Cruz ZC, et al. Clinicopathologic studies of age-related macular degeneration with classic subfoveal choroidal neovascularization treated with photodynamic therapy. Retina 2001; 21(5): 478–86
Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, et al. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol 2002 Jun; 120(6): 835–44
Schlötzer-Schrehardt U, Viestenz A, Naumann GOH, et al. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol 2002 Sep; 240(9): 748–57
Schmidt-Erfurth U, Miller J, Sickenberg M, et al. Photodynamic therapy of subfoveal choroidal neovascularization: clinical and angiographic examples. Graefes Arch Clin Exp Ophthalmol 1998; 236: 365–74
Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization by age-related macular degeneration: results of a single treatment in a phase I and 2 study. Arch Ophthalmol 1999; 117: 1161–73
Chen JC, Marinier JA, Kergoat H, et al. Choroidal blood flow in verteporfin treatment of age related macular degeneration [abstract]. Invest Ophthalmol Vis Sci 2002; 43: E586
Schmidt-Erfurth U, Miller JW, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of retreatment in a phase 1 and 2 study. Arch Ophthalmol 1999; 117: 1177–87
Houle J-M, Strong A. Clinical pharmacokinetics of verteporfin. J Clin Pharmacol 2002 May; 42(5): 547–57
Richter AM, Waterfield E, Jain AK, et al. Liposomal delivery of a photosensitizer, benzoporphyrin derivative monoacid ring A (BPD), to tumor tissue in a mouse tumor model. Photochem Photobiol 1993; 57: 1000–6
Novartis. Prescribing information: Visudyne (verteporfin for injection) [online]. Available from URL: http://www.visudyne.com [Accessed 2003 May 12]
Treatment of Age-related Macular Degeneration with Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one year results of 2 randomized clinical trials - TAP Report 1. Arch Ophthalmol 1999; 117: 1329–45
Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials - TAP Report 2. Arch Ophthalmol 2001 Feb; 119(2): 198–207
Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes -TAP Report no. 3. Arch Ophthalmol 2002 Nov; 120(11): 1443–54
Rubin GS, Bressler NM, and the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effects of verteporfin therapy on contrast on sensitivity: results from the Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Investigation - TAP Report no. 4. Retina 2002 Oct; 22(5): 536–44
Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: three-year results of an open-label extension of 2 randomized clinical trials — TAP Report no. 5. Arch Ophthalmol 2002 Oct; 120(10): 1307–14
Singerman LJ. Two-year results of an open-label extension of the TAP Investigation evaluating verteporfin therapy for subfoveal CNV due to AMD [abstract]. TAP Study Group. Retina 2002 Congress; 2002 Sep 28–Oct 2; San Francisco
Kaiser PK, and TAP Study Group. Five-year results of verteporfin therapy for subfoveal CNV due to AMD: third year of an open-label extension of the TAP Investigation [abstract]. Invest Ophthalmol Vis Sci 2003; 44: E1099
QLT Inc. New clinical data confirm role of visudyne (verteporfin) therapy as standard of care in choroidal neovascularization (CNV) due to age-related macular degeneration [media release]. 2003 Sep 18
Sharma S. Update in retina: photodynamic therapy for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Can J Ophthalmol 2001 Feb; 36(1): 7–10
Wormald R, Evans J, Smeeth L. Photodynamic therapy for neovascular age-related macular degeneration (Cochrane Review). Available in the Cochrane Library [database on disk and CD ROM]. Updated quarterly. The Cochrane Collaboration; issue 3. Oxford: Update Software, 2003: CD002030
Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization — Verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001 May; 131: 541–60
Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) and Verteporfin In Photodynamic Therapy (VIP) Study Groups. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP Report no. 1. Am J Ophthalmol 2003 Sep; 136(3): 407–18
Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial — VIP Report no. 1. Ophthalmology 2001 May; 108(5): 841–52
Verteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial - VIP Report no. 3. Ophthalmology 2003; 110: 667–73
Saperstein DA, Rosenfeld PJ, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin in the ocular histoplasmosis syndrome: one-year results of an uncontrolled, prospective case series. Ophthalmology 2002 Aug; 109(8): 1499–505
Saperstein DA. Effects over two years of verteporfin therapy on choroidal neovascularization secondary to the ocular histoplasmosis syndrome (VOH study) [abstract]. VOH Study Group. Invest Ophthalmol Vis Sci 2002 May; 43: E982
Smith DH, Drummond M, Fenn P. Modelling the long-term benefits of photodynamic therapy (PDT) with verteporfin for age-related macular degeneration (AMD) Discussion paper 187 [online]. Available from URL: http://www.york.ac.uk/inst/che/DP187full.pdf [Accessed 2003 Apr 7]
Smith DH, Fenn P, Drummond M. Cost effectiveness of photodynamic therapy (PDT) with verteporfin in the UK [abstract no. PES6]. Value Health 2002; 5: 247
Sharma S, Brown GC, Brown MB, et al. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 2001; 108: 2051–9
Beaumont P, Singerman LJ, Lees M, et al. Economic evaluation of photodynamic therapy with verteporfin for predominantly classic subfoveal CNV secondary to AMD [abstract]. Retina 2002 Congress; 2002 Sep 28–Oct 2; San Francisco
Lees M, Davey PJ, Price N, et al. Economic evaluation of photodynamic therapy with verteporfin for small subfoveal CNV lesions: the case of Australia [abstract no. PES6]. Value Health 2003; 6(3): 239
The European Agency for the Evaluation of Medicinal Products (EMEA). Summary of product characteristics: visudyne [online]. Available from URL: http://www.eudra.org/humandocs/Humans/EPAR/Visudyne/Visudyne.htm [Accessed 2003 May 12]
QLT Inc. Commercial: visudyne — approval status [online]. Available from URL: http://www.qtlinc.com [Accessed 2003 Sep 19]
Novartis/QLT Visudyne expanded indications cover up to 250,000 patients. Pink Sheet 2001 Aug 27; 63(35): 18
Visudyne infusion. Pharm J 2000 Aug 5; 265: 213
Novartis Pharmaceutical Canada Inc. Visudyne prescribing information [online]. Available from URL: http://www.pharma.ca.novartis.com/e/products/visudyne.shtml [Accessed 2003 Sep 19]
Novartis Pharmaceuticals Australia Pty Ltd. Visudyne (verteporfin) prescribing information [online]. Available from URL: http://www.novartis.com.au/healthcare_professionals.html [Accessed 2003 Sep 19]
Acknowledgements
The full text article in Drugs 2003; 63 (22): 2521–2554 was reviewed by: P. Kaiser, The Cleveland Clinic Foundation, Cole Eye Institute, Cleveland, Ohio, USA; M. Potter, University of British Columbia, Department of Ophthalmology, Vancouver, British Columbia, Canada; S. Sharma, Queen’s University, Department of Ophthalmology, Kingston, Ontario, Canada; M. Stur, University of Vienna Medical School, Allegemeines Krankenhaus, Department of Ophthalmology, Vienna, Austria; S. Wolf, Klinik und Poliklinik für Augenheilkunde, Leipzig, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This Spotlight is derived from abstract and summary text of an Adis Drug Evaluation originally published in Drugs 2003; 63 (22): 2521–2554. Reviewers of the original full text article are listed in the Acknowledgements section.
Rights and permissions
About this article
Cite this article
Keam, S.J., Scott, L.J. & Curran, M.P. Spotlight on Verteporfin in Subfoveal Choroidal Neovascularisation. Drugs Aging 21, 203–209 (2004). https://doi.org/10.2165/00002512-200421030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200421030-00005