Abstract
Background: Adverse drug reactions (ADRs) contribute significantly to patient morbidity and mortality, as well as to costs for healthcare systems. Our aim was to evaluate the type and incidence of ADRs in a paediatric hospital population, comparatively ascertained by two different methodological approaches.
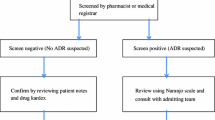
Methods: Our prospective study enrolled all patients admitted to two of the general children wards (46 beds) and the paediatric intensive care unit (6 beds) at the HELIOS Klinikum Wuppertal teaching hospital in Germany, over the study period of 3 months. We used two methods to detect ADRs. The intensified surveillance system relied on a trained physician conducting ward rounds and assessing patient charts. The computer-assisted screening of pathological laboratory parameters used values slightly below or above the age-specific normal range as a trigger signal for a potential ADR, which was subsequently assessed by trained personnel.
Results: By applying both methods simultaneously we observed that 14.1% of children experienced an ADR while they were hospitalised and 2.7% of children were admitted to hospital because of the ADR. Intensified surveillance resulted in the detection of 101 ADRs in 11.9% of patients, predominantly presenting with gastrointestinal symptoms, skin and CNS disorders; computer-assisted screening identified 45 ADRs in 5.7% of patients, mainly with drug-induced blood dyscrasia and liver damage. Furthermore, the ADRs detected by the intensified method were more severe, affected younger children and showed a closer causal attributability to the reaction than the ADRs observed by the computerised method. The spectra of drugs involved were similar, with the anti-infectives being suspected most frequently. The sensitivities of the intensified surveillance system and the computerised surveillance screening came to 67.2% and 44.8%, respectively, with computer-assisted screening having a specificity of 72.8%. The mean positive predictive value of the pathological laboratory values under surveillance by computer-assisted screening was 18.6%. Approximately 25% of ADR-related drugs administered were used for off-label indications.
Conclusion: Using the published literature for comparison, we found that ADRs occur as frequently in paediatric patients as in adult patients. Intensified surveillance and computerised surveillance applied in the paediatric setting show substantial differences in their detection specificities. A higher number of and more severe ADRs can be detected by intensified surveillance than by computerised surveillance, but require higher personnel resources.






Similar content being viewed by others
References
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997; 277: 307–11
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–6
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–5
Impicciatore P, Choonara I, Clarkson A, et al. Incidence of adverse drug reactions in pediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol 2001; 52: 77–83
McKenzie MW, Stewart RB, Weiss CF, et al. A pharmacist-based study of the epidemiology of adverse drug reactions in pediatric medicine patients. Am J Hosp Pharm 1973; 30: 898–903
Whyte J, Greenan E. Drug usage and adverse drug reactions in pediatric patients. Acta Paediatr Scand 1977; 66: 767–75
Mitchell AA, Goldman P, Shapiro S, et al. Drug utilization and reported adverse reactions in hospitalized children. Am J Epidemiol 1979; 110: 196–204
Choonara IA, Harris F. Adverse drug reactions in medical inpatients. Arch Dis Child 1984; 59: 578–80
Easton KL, Parsons BJ, Starr M, et al. The incidence of drug-related problems as a cause of hospital admissions in children. Med J Aust 1998; 169: 356–9
Martinez-Mir I, Garcia-Lopez M, Palop V, et al. A prospective study of adverse drug reactions as a cause of admission to a pediatric hospital. Br J Clin Pharmacol 1996; 42: 319–24
Thuermann P. Methods and systems to detect adverse drug reactions in hospital. Drug Saf 2001; 24: 961–8
Dormann H, Muth-Selbach U, Krebs S, et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf 2000; 22: 161–8
Levy M, Azaz-Livshits T, Sadan B, et al. Computerized survelliance of adverse drug reactions in hospital: implementation. Eur J Clin Pharmacol 1999; 54: 887–92
Classen DC, Pestotnik SL, Evans RS, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991; 266: 2847–51
Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA 1998; 280: 1317–20
Impicciatore P, Choonara I. Status of new medicines approved by the European Medicines Evaluation Agency regarding pediatric use. Br J Clin Pharmacol 1999; 48: 15–8
Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in pediatric wards in European countries. European Network for Drug Investigation in Children. BMJ 2000; 320: 79–82
Turner S, Nunn AJ, Fielding K, et al. Adverse drug reactions to unlicensed and off-label drugs on pediatric wards: a prospective study. Acta Paediatr 1999; 88: 965–8
Weiss J, Krebs S, Hoffmann C, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics 2002; 110: 254–7
Thuermann PA, Windecker R, Steffen J, et al. Detection of adverse drug reactions in a neurological department: comparison between intensified surveillance and a computer-assisted approach. Drug Saf 2002; 25: 713–24
Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol 1990; 11: 272–6
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–9
BPI Service GmbH: Fach Info-Service (SPCs) Deutschland. Bundesverband der Pharmazeutischen Industrie [online]. Available from URL: http://www.fachinfo.de [Accessed 2004 Feb 24]
Thomson Corporation. Thomson Micromedex® Healthcare Series 2001, Vol. 108, 2001 [online]. Available from URL: http://www.micromedex.com [Accessed 2004 Feb 24]
Schosser R, Quast U. Verdacht auf Nebenwirkungen: Medizinische Uberlegungen zur Kausalitat. Pharm Ind 1998; 60: 185–91
Rawlins MD, Thompson JW. Mechanisms of adverse drug reactions. In: Davies DM, editor. Textbook of adverse drug reactions. Oxford: Oxford University Press, 1991: 18–45
Vazquez de la Villa A, Luna del Castillo JD, Galdo Munoz G, et al. Adverse reactions caused by drugs in pediatrics [Spanish]. An Esp Pediatr 1989; 31: 49–53
Gonzalez-Martin G, Caroca CM, Paris E. Adverse drug reactions (ADRs) in hospitalized pediatric patients: a prospective study. Int J Clin Pharmacol Ther 1998; 36: 530–3
Martinez-Mir I, Garcia-Lopez M, Palop V, et al. A prospective study of adverse drug reactions in hospitalized children. Br J Clin Pharmacol 1999; 47: 681–8
Mitchell AA, Lacouture PG, Sheehan JE, et al. Adverse drug reactions in children leading to hospital admission. Pediatrics 1988; 82: 24–9
Yosselson-Superstine S, Weiss T. Drug-related hospitalization in pediatric patients. J Clin Hosp Pharm 1982; 7: 195–203
McKenzie MW, Marchall GL, Netzloff ML, et al. Adverse drug reactions leading to hospitalization in children. J Pediatr 1976; 89: 487–90
Bonati M, Marchetti F, Zullini MT, et al. Adverse drug reactions in neonatal intensive care units. Adverse Drug React Acute Poisoning Rev 1990; 9: 103–18
Aranda JV, Portuguez-Malavasi A, Collinge JM, et al. Epidemiology of adverse drug reactions in the newborn. Dev Pharmacol Ther 1982; 5: 173–84
Gill AM, Leach HJ, Hughes J, et al. Adverse drug reactions in a pediatric intensive care unit. Acta Paediatr 1995; 84: 438–41
Dharnidharka VR, Kandoth P. Pediatric inpatient morbidity patterns and drug usage in a teaching hospital serving an underdeveloped area. Indian J Public Health 1999; 43: 64–6
Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001; 285: 2114–20
Thomas EJ, Lipsitz SR, Studdert DM, et al. The reliability of medical record review for estimating adverse event rates. Ann Intern Med 2002; 136: 812–6
Kilbridge P. Computer crash -lessons from a system failure. N Engl J Med 2003; 348: 881–2
Horen B, Montastruc JL, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in pediatric outpatients. Br J Clin Pharmacol 2002; 54: 665–70
Neubert A, Dormann H, Weiss J, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf 2004; 27: 1059–67
t’Jong GW, van der Linden PD, Bakker EM, et al. Unlicensed and off-label drug use in a pediatric ward of a general hospital in the Netherlands. Eur J Clin Pharmacol 2002; 58: 293–7
Andrews LB, Stocking C, Krizek T, et al. An alternative strategy for studying adverse events in medical care. Lancet 1997; 349: 309–13
Acknowledgements
Steffen Haffner and Nicoletta von Laue contributed equally to this article. We thank Ute Tenter and Erich Reese for their technical contribution to the study.
The results of this publication are part of the thesis of Nicoletta von Laue, which has been submitted in fulfilment of the requirements for the degree of Doctor of Medicine at the University of Witten/Herdecke, Germany.
No sources of funding were used to assist in the preparation of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haffner, S., von Laue, N., Wirth, S. et al. Detecting Adverse Drug Reactions on Paediatric Wards. Drug-Safety 28, 453–464 (2005). https://doi.org/10.2165/00002018-200528050-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200528050-00008