Abstract
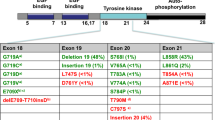
We report the case of a male Mongolian lifelong non-smoker with recurrent non-small-cell lung cancer (NSCLC) who developed resistance to the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib after initially responding to this agent but then subsequently had another response to a second course of erlotinib treatment after intervening gemcitabine chemotherapy. Sixteen months after the patient received chemoradiotherapy with gemcitabine/cisplatin plus radiotherapy, his recurrent mediastinal metastases were found to have progressed. Treatment with erlotinib was followed by an initial, partial response but evidence of progression was again observed 6 months later. The patient was then treated with gemcitabine chemotherapy, which resulted in a reduction in tumour volume. One month later, progression of mediastinal metastases was again observed and the patient received a second course of erlotinib. Another partial response occurred and the patient’s disease remained stable at the 9-month follow-up visit (and with no reported symptom progression at an 11-month telephone follow-up). Genetic examination of tumour tissue collected at the time of the original diagnosis and during the second course of erlotinib therapy revealed activating exon 19 mutation in the EGFR gene. This case suggests that resistance to erlotinib may change following chemotherapy and that repeat erlotinib therapy may be worth considering after chemotherapy in NSCLC patients who initially respond positively to erlotinib treatment but subsequently experience recurrence of disease.

Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008 Mar–Apr; 58(2): 71–96
Pujol JL, Barlesi F, Daures JP. Should chemotherapy combinations for advanced non-small cell lung cancer be platinum-based? A meta-analysis of phase III randomized trials. Lung Cancer 2006; 51: 335–45
Akita H. Second-line treatment for advanced non-small-cell lung cancers [in Japanese]. Gan To Kagaku Ryoho 2005; 32: 15–8
Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001 Sep; 37Suppl. 4: S3–8
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005 Jul 14; 353(2): 123–32
Scagliotti GV. Potential role of multi-targeted tyrosine kinase inhibitors in non-small-cell lung cancer. Ann Oncol 2007 Sep; 18Suppl. 10: x32–41
Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–81
Jancarikova D, Pesek M, Benesova L, et al. Acquired resistance of pulmonary adenocarcinoma to initially successful targeted therapy due to EGFR mutation T790M. Anti-cancer Res 2007 Jul–Aug; 27(4A): 1879–82
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005 Feb 24; 352(8): 786–92
Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009 Aug; 28Suppl. 1: S24–31
Response Evaluation Criteria In Solid Tumors (RECIST) [online]. Available from URL: http://www.recist.com/ index.html [Accessed 2010 Aug 1]
Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010 Jan 10; 28(2): 357–60
Katzel JA, Fanucchi MP, Li Z. Recent advances of novel targeted therapy in non-small cell lung cancer. J Hematol Oncol 2009; 2: 2
Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008; 14: 2895–9
Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007 Dec 26; 104(52): 20932–7
Acknowledgements
This case was partially supported by the Research Fund of Shandong Provincial Health Bureau 2007QW036, the Doctor Fund Project of Shandong Province BS2009YY012 and NSFC30700196. The funding source had no involvement in the design; collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit this article for publication. The authors have no conflicts of interest that are directly relevant to the content of this case report. The manuscript was kindly edited by Paul Stanton (University of Michigan, MI, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, J., Liu, H. et al. Erlotinib Resistance is Altered after Gemcitabine Chemotherapy for Recurrent Non-Small-Cell Lung Cancer. Clin. Drug Investig. 31, 279–283 (2011). https://doi.org/10.2165/00044011-200930100-8
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200930100-8