Abstract
Since renal failure itself creates an immunocompromised situation, malignant tumours in haemodialysis patients are increasing due to the prolonged lifespan of these patients. In treating these patients with anticancer agents, dosage reduction is often recommended to avoid adverse drug reactions, particularly for drugs with extensive renal excretion. On the other hand, if an anticancer drug is removed significantly by haemodialysis, dosage increase would be required to ensure adequate therapeutic efficacy. We address in this review the clinical pharmacokinetic aspects of antineoplastic therapy, and the application of pharmacokinetic principles to the adjustment of dosage of anticancer agents in haemodialysis patients.




Similar content being viewed by others
References
Matas AJ, Simmons RL, Kjellstrand CM, et al. Increased incidence of malignancy during chronic renal failure. Lancet 1975; I: 883–6
Miach PJ, Dawborn JK, Xipell J. Neoplasia in patients with chronic renal failure on long-term dialysis. Clin Nephrol 1976; 5: 101–4
Sutherland GA, Glass J, Gabriel R. Increased incidence of malignancy in chronic renal failure. Nephron 1977; 18: 182–4
Sauer H, Fuger K, Blumenstein M. Modulation of cytotoxicity of cytostatic drugs by hemodialysis in vitro and in vivo. Cancer Treat Rev 1990; 17: 293–300
Gwilt PR, Perrier D. Plasma protein binding and distribution characteristics of drugs as indices of their hemodialysability. Clin Pharmacol Ther 1978; 24: 154–61
Keller F, Wilms H, Schultze G, et al. Effect of plasma protein binding, volume of distribution and molecular weight on the fraction of drugs eliminated by hemodialysis. Clin Nephrol 1983; 19: 205–10
Lee CS, Marbury TC. Drug therapy in patients undergoing hemodialysis: clinical pharmacokinetic considerations. Clin Pharmacokinet 1984; 9: 42–66
Winchester JF. Evolution of artificial organs/extracorporeal removal of drugs. Artif Organs 1986; 10: 316–23
Wani MC, Taylor HL, Wall ME, et al. Plant antitumor agents: VI. the isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93: 2325–7
Cresteil T, Monsarrat B, Alvinerie P, et al. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res 1994; 54: 386–92
Kumar GN, Walle UK, Walle T. Cytochrome P450 3A-mediated human liver microsomal taxol 6 alpha-hydroxylation. J Pharmacol Exp Ther 1994; 268: 1160–5
Harris JW, Rahman A, Kim BR, et al. Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res 1994; 54: 4026–35
Wiernik PH, Schwartz EL, Stemmati JJ, et al. Phase I clinical and pharmacokinetic study of taxol. Cancer Res 1987; 47: 2486–93
Longnecker SM, Donehower RC, Cates AE, et al. Highperformance liquid Chromatographic assay for taxol in human plasma and urine and pharmacokinetics in a phase I trial. Cancer Treat Rep 1987; 71: 53–9
Woo MH, Gregornik D, Shearer PD, et al. Pharmacokinetics of paclitaxel in an anephric patient. Cancer Chemother Pharmacol 1999; 43: 92–6
Tornita M, Kurata H, Aoki Y, et al. Pharmacokinetics of paclitaxel and cisplatin in a hemodialysis patient with recurrent ovarian cancer. Anticancer Drugs 2001; 12: 485–7
Watanabe M, Aoki Y, Tornita M, et al. Paclitaxel and carboplatin combination chemotherapy in a hemodialysis patient with advanced ovarian cancer. Gynecol Oncol 2002; 84: 335–8
Ohtsu T, Sasaki Y, Tamura T, et al. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion. Clin Cancer Res 1995; 1: 599–606
Huizing MT, Keung AC, Rosing H, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol 1993; 11: 2127–35
Balat O, Kudelka AP, Edwards CL, et al. A case report of paclitaxel administered to a patient with platinum-refractory ovarian cancer on long-term hemodialysis. Eur J Gynaecol Oncol 1996; 17: 232–3
Jeyabalan N, Hirte HW, Moens F. Treatment of advanced ovarian carcinoma with carboplatin and paclitaxel in a patient with renal failure. Int J Gynecol Cancer 2000; 10: 463–8
De Conti RC, Toftness BR, Lange RC, et al. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res 1973; 33: 1310–5
Gouyette A, Lemoine R, Adhemar JP, et al. Kinetics of cisplatin in an anuric patient undergoing hemofiltration dialysis. Cancer Treat Rep 1981; 65: 665–8
Tanabe N, Goto M, Morita H, et al. Pharmacokinetics of cisdiammine-dichlor-platin in a hemodialysis patient. Cancer Invest 1991; 9: 629–35
Ribrag V, Droz JP, Morizet J, et al. Test dose-guided administration of cisplatin in an anephric patient: a case report. Ann Oncol 1993; 4: 679–82
Gorodetsky R, Vexler A, Bar-Khaim Y, et al. Plasma platinum elimination in a hemodialysis patient treated with cisplatin. Ther Drug Monit 1995; 17: 203–6
Ayabe H, Uchikawa T, Kimino K, et al. Pharmacokinetics of cisplatin and vindesine in a patient with chrnoic renal failure undergoing hemodialysis. Gan To Kagaku Ryoho 1989; 16: 3283–5
Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7: 1748–56
Egorin MJ, Van Echo DA, Tipping SJ, et al. Pharmacokinetics and dosage reduction of cis-diammine (1,1-cyclobutanedicarboxylato) platinum in patients with impaired renal function. Cancer Res 1984; 44: 5432–8
Gormley PE, Bull JM, LeRoy AF, et al. Kinetics of cisdichlorodiammineplatinum. Clin Pharmacol Ther 1979; 25: 351–7
Takahashi K, Seki T, Nishikawa K, et al. Antitumor activity and toxicity of serum protein-bound platinum formed from cisplatin. Jpn J Cancer Res 1985; 76: 68–74
Motzer RJ, Niedzwiecki D, Isaacs M, et al. Carboplatin-based chemotherapy with pharmacokinetic analysis for patients with hemodialysis-dependent renal insufficiency. Cancer Chemother Pharmacol 1990; 27: 234–8
Koren G, Weitzman S, Klein J, et al. Comparison of carboplatin pharmacokinetics between an anephric child and two children with normal renal function. Med Pediatr Oncol 1993; 21: 368–72
Chatelut E, Rostaing L, Gualano V, et al. Pharmacokinetics of carboplatin in a patient suffering from advanced ovarian carcinoma with hemodialysis-dependent renal insufficiency. Nephron 1994; 66: 157–61
Kurata H, Yoshiya N, Ikarashi H, et al. Pharmacokinetics of carboplatin in a patient under hemodialysis. Gan To Kagaku Ryoho 1994; 21: 547–50
Yanagawa H, Takishita Y, Bando H, et al. Carboplatin-based chemotherapy in patients undergoing hemodialysis. Anticancer Res 1996; 16: 533–5
Suzuki S, Koide M, Sakamoto S, et al. Pharmacokinetics of carboplatin and etoposide in a haemodialysis patient with Merkel-cell carcinoma. Nephrol Dial Transplant 1997; 12: 137–40
Obana T, Tanio Y, Takenaka M, et al. Chemotherapy for smallcell lung cancer (SCLC) patients with renal failure. Gan To Kagaku Ryoho 2002; 29: 435–8
Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum (II) in patients with normal and impaired renal function. Cancer Res 1984; 44: 1693–7
Sinkule JA. Etoposide: a semisynthetic epipodophyllotoxin: chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy 1984; 4: 61–73
O’Dwyer PJ, Leyland-Jones B, Alonso MT, et al. Etoposide (VP-16-213): current status of an active anticancer drug. N Engl J Med 1985; 312: 692–700
Allen LM, Creaven PJ. Comparison of the human pharmacokinetics of VM-26 and VP-16, two antineoplastic epipodophyllotoxin glucopyranoside derivatives. Eur J Cancer 1975; 11: 697–707
Evans WE, Sinkule JA, Crom WR, et al. Pharmacokinetics of teniposide (VM26) and etoposide (VP16-213) in children with cancer. Cancer Chemother Pharmacol 1982; 7: 147–50
D’Incaici M, Rossi C, Zucchetti M, et al. Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res 1986; 46: 2566–71
Miyaoka K, Matsubara T, Matsumoto T, et al. Pharmacokinetic study of etoposide in aged patient with non Hodgkin lymphoma receiving hemodialysis. Gan To Kagaku Ryoho 1991; 18: 2325–8
Holthuis JJ, Van de Vyver FL, van Oort WJ, et al. Pharmacokinetic evaluation of increasing dosages of etoposide in a chronic hemodialysis patient. Cancer Treat Rep 1985; 69: 1279–82
Ono S, Miyazaki T, Nishikawa K, et al. Etoposide and cisplatin combination chemotherapy in a patient with small cell lung carcinoma under artificial hemodialysis. Gan To Kagaku Ryoho 1992; 19: 115–8
English MW, Lowis SP, Peng B, et al. Pharmacokinetically guided dosing of carboplatin and etoposide during peritoneal dialysis and haemodialysis. Br J Cancer 1996; 73: 776–80
Chirstophidis N, Vajda FJ, Lucas I, et al. Fluorouracil therapy in patients with carcinoma of the large bowel: a pharmacokinetic comparison of various rates and routes of administration. Clin Pharmacokinet 1978; 3: 330–6
Cohen JL, Irwin LE, Marshall GJ, et al. Clinical pharmacology of oral and intravenous 5-fluorouracil (NSC-19893). Cancer Chemother Rep 1974; 58: 723–31
Heggie GD, Sommadossi JP, Cross DS, et al. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 1987; 47: 2203–6
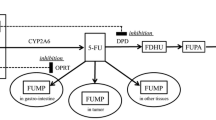
Rengelshausen J, Hull WE, Schwenger V, et al. Pharmacokinetics of 5-fluorouracil and its catabolites determined by 19F nuclear magnetic resonance spectroscopy for a patient on chronic hemodialysis. Am J Kidney Dis 2002; 39: E10
Molina R, Fabian C, Cowley Jr B. Use of charcoal hemoperfusion with sequential hemodialysis to reduce serum methotrexate levels in a patient with acute renal insufficiency. Am J Med 1987; 82: 350–2
Relling MV, Stapleton FB, Ochs J, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer 1988; 62: 884–8
Gauthier E, Gimonet JF, Piedbois P, et al. Effectiveness of hemodialysis in a case of acute methotrexate poisoning. Presse Med 1990; 19: 2023–5
Thomson AH, Daly M, Knepil J, et al. Methotrexate removal during haemodialysis in a patient with advanced laryngeal carcinoma. Cancer Chemother Pharmacol 1996; 38: 566–70
Grochow LB, Colvin M. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 1979; 4: 380–94
Busse D, Busch FW, Bohnenstengel F. Dose escalation of cyclophosphamide in patients with breast cancer: consequences for pharmacokinetics and metabolism. J Clin Oncol 1997; 15: 1885–96
Bagley CM, Bostick FW, De Vita VT. Clinical pharmacology of cyclophosphamide. Cancer Res 1973; 33: 226–33
Chen TL, Kennedy MJ, Anderson LW. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Dispos 1997; 25: 544–51
Moore MJ Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 1991; 20: 194–208
Bramwell V, Calvert RT, Edwards G. The disposition of cyclophosphamide in a group of myeloma patients. Cancer Chemother Pharmacol 1979; 3: 253–9
Juma FD, Rogers HJ, Trounce JR. Effect of renal insufficiency on the pharmacokinetics of cyclophosphamide and some of its metabolites. Eur J Clin Pharmacol 1981; 19: 443–51
Haubitz M, Bohnenstengel F, Brunkhorst R, et al. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int 2002; 61: 1495–501
Mouridsen HT, Jacobsen E. Pharmacokinetics of cyclophosphamide in renal failure. Acta Pharmacol Toxicol 1975; 36: 409–14
Wang LH, Lee CS, Majeske BL, et al. Clearance and recovery calculations in hemodialysis: application to plasma, red blood cells, and dialysate measurements for cyclophosphamide. Clin Pharmacol Ther 1981; 29: 365–72
Milsted RAV, Jarman M. Hemodialysis during cyclophosphamide treatment. BMJ 1978; 1: 820–1
Perry JJ, Fleming RA, Rocco MV, et al. Administration and pharmacokinetics of high-dose cyclophosphamide with hemodialysis support for allogeneic bone marrow transplantation in acute leukemia and end-stage renal disease. Bone Marrow Transplant 1999; 23: 839–42
Bischoff ME, Blau W, Wagner T, et al. Total body irradiation and cyclophosphamide is a conditioning regimen for unrelated bone marrow transplantation in a patient with chronic myelogenous leukemia and renal failure on hemodialysis. Bone Marrow Transplant 1998; 22: 591–3
Carlson L, Goren MP, Bush DA, et al. Toxicity, pharmacokinetics, and in vitro hemodialysis clearance of ifosfamide and metabolites in an anephric pediatric patient with Wilms’ tumor. Cancer Chemother Pharmacol 1998; 41: 140–6
Skinner R, Sharkey IM, Pearson AD, et al. Ifosfamide, mesna, and nephrotoxicity in children. J Clin Oncol 1993; 11: 173–90
Rossi R, Godde A, Kleinebrand A, et al. Unilateral nephrectomy and cisplatin as risk factors of ifosfamide-induced nephrotoxicity: analysis of 120 patients. J Clin Oncol 1994; 12: 159–65
Seyffart G. Drugs in renal failure: dosing guidelines for frequently used drugs in end-stage renal disease and dialysis patients. Blood Purif 1985; 3: 140–68
Benner W, Aronoff GR, Morrison G, et al. Drug prescribing in renal failure: dosing guidelines for adults. Am J Kidney Dis 1983; 3: 155–93
Acknowledgements
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomita, M., Aoki, Y. & Tanaka, K. Effect of Haemodialysis on the Pharmacokinetics of Antineoplastic Drugs. Clin Pharmacokinet 43, 515–527 (2004). https://doi.org/10.2165/00003088-200443080-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200443080-00002