Abstract
Prostaglandin I2 (PGI2) analog is regarded as a potential candidate for treating asthma. Human myeloid dendritic cells (mDCs) play a critical role in the pathogenesis of asthma. However, the effects of PGI2 analog on human mDCs are unknown. In the present study, circulating mDCs were isolated from six healthy subjects. The effects of PGI2 analogs iloprost and treprostinil on cytokine production, maturation and T-cell stimulatory function of human mDCs were investigated. Tumor necrosis factor (TNF)-α and interleukin (IL)-10 were measured by enzyme-linked immunosorbent assay. The expression of costimulatory molecules was investigated by flow cytometry. T-cell stimulatory function was investigated by measuring interferon (IFN)-γ, IL-13 and IL-10 production by T cells cocultured with iloprost-treated mDCs. Intracellular signaling was investigated by Western blot and chromatin immunoprecipitation. We found that iloprost and treprostinil induced IL-10, but suppressed TNF-α production in polyinosinic-polycytidylic acid (poly I:C)-stimulated mDCs. This effect was reversed by the I-prostanoid (IP), E-prostanoid (EP) receptor antagonists or intracellular free calcium (Ca2+) chelator. Forskolin, an adenyl cyclase activator, conferred a similar effect. Iloprost and treprostinil increased intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) levels, and iloprost also increased intracellular Ca2+. Iloprost suppressed poly I:C-induced mitogen-activated protein kinase (MAPK) phospho-p38 and phospho-activating transcription factor (ATF)2 expression. Iloprost downregulated poly I:C-induced histone H3K4 trimethylation in the TNFA gene promoter region via suppressing translocation of histone 3 lysine 4 (H3K4)-specific methyltransferases MLL (mixed lineage leukemia) and WDR5 (WD repeat domain 5). Iloprost-treated mDCs inhibited IL-13, IFN-γ and IL-10 production by T cells. In conclusion, PGI2 analogs enhance IL-10 and suppress TNF-α expression through the IP/EP2/EP4 receptors-cAMP and EP1 receptor-Ca2+ pathway. Iloprost suppressed TNF-α expression via the MAPK-p38-ATF2 pathway and epigenetic regulation by downregulation of histone H3K4 trimethylation.
Similar content being viewed by others
Introduction
Asthma is a chronic airway inflammatory disorder with accumulation of inflammatory cells including eosinophils, lymphocytes, neutrophils and mast cells. The disease process is regulated by the cytokines and chemokines and also by the interactions between the antigen-presenting cells and T cells (1). Tumor necrosis factor (TNF)-α, a pleiotropic proinflammatory cytokine, is increased in TNF-α mRNA and protein levels in the airways of asthmatic patients (2). Emerging evidence suggests the central role of TNF-α in asthma for its properties of developing mast cell-mediated airway hyperresponsiveness, activating eosinophil proliferation and regulating chemokine production in monocytes (3). Recent studies suggest the particular role of TNF-α in severe refractory asthma according to its properties of neutrophil recruitment, induction of resistance to steroid and involvement of airway remodeling (4). Interleukin (IL)-10 is a broad antiinflammatory cytokine functioning as a feedback regulation of T helper (Th) 1 and Th2 responses (5). IL-10 inhibits survival and cytokine production of inflammatory cells and can limit allergic airway inflammation and hyperreactivity (6). IL-10-deficient mice express highly elevated levels of Th2 cytokine after allergen challenging and exhibit exaggerated airway inflammation (7). In contrast to TNF-α, the level of IL-10 in the lungs of asthmatic patients is significantly decreased (8).
Dendritic cells (DCs) are professional antigen-presenting cells and are highly heterogeneous in terms of origin, morphology, phenotype and function. DCs play a major role in initiation and regulation of adaptive immune responses to the stimulation of antigens and allergens (9). In a murine asthma model, myeloid dendritic cells (mDCs) accumulate in the allergen-challenged airways during the acute phase, and the depletion of mDCs attenuates the airway inflammation and hyperresponsiveness (10). In human asthma, mDCs accumulate in bronchoalveolar lavage fluid after an allergen challenge (11), and the influx of mDCs into the airways can be augmented by endotoxins (12). The induction and maintenance of inflammatory responses to allergens in persistent airway disease needs the involvement of mDCs (13). These data suggested the critical role of mDCs in allergic airway inflammation.
Prostaglandins are generally regarded as proinflammatory molecules. However, prostaglandin I2 (PGI2) was recently shown to exhibit some antiinflammatory functions (14). Because PGI2 is very unstable, PGI2 analogs with more chemical stability have been used in clinical application. Iloprost, a stable PGI2 analog, is a well-accepted medication for human pulmonary arterial hypertension acting as a vasodilator. In murine asthma model, signaling via the I-prostanoid (IP) receptor by iloprost suppresses the cardinal features of asthma via inhibition of lung DC maturation and migration to regional lymph nodes (15). Our previous work demonstrated that iloprost can modulate cytokine expression via the IP receptor in human plasmacytoid DCs (16). However, the effects of PGI2 analogs on human mDCs are still not elucidated.
Epigenetic regulation, including acetylation of core histones by histone acetyltransferase or histone deacetylase, has been shown to be involved in inflammatory expression in monocytes and macrophages (17). In asthmatic patients, the histone acetyltransferase activity is markedly increased, whereas the histone deacetylase activity is reduced, resulting in the overexpression of inflammatory genes (18,19). Recently, we showed that epigenetic regulation is an important mechanism by which iloprost modulates asthma-related chemokines expression in monocytes (20). In the present study, we examined the in vitro effect of two commonly used PGI2 analogs, iloprost and treprostinil, on the expression of cytokines by mDCs and also investigate the intracellular mechanism including epigenetic regulation. The effects of PGI2 analogs on the expression of costimulatory molecules and the T-cell stimulatory functions of mDCs were also studied.
Materials and Methods
Isolation and Culture of mDCs
The study protocol was approved by the Institution Review Board of Kaohsiung Medical University Hospital. Peripheral blood samples (250 mL) were obtained from healthy and nonsmoking individuals who had no history of allergic or systemic disease (n = 6) after gaining informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Histopaque (Pharmacia Biotech, Uppsala, Sweden) and then separated into a low-density fraction enriched in DCs by centrifuging for 30 min at 300g. Blood mDCs were magnetically sorted from PBMCs using blood DC antigen (BDCA-1) cell isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s instructions. Isolated mDCs were >90% in purity. Purified mDCs were cultured in 24-well round-bottom plates (105/well) in 500 µL RPMI 1640 buffered with NaHCO3, containing 10% heat-inactivated endotoxin-tested fetal calf serum, 100 IU/mL penicillin and 0.1 mg/mL streptomycin. Isolated mDCs were treated with varying doses of iloprost (10−8 to 10−7 mol/L) or treprostinil (10−9 to 10−7 mol/L) or vehicle solution for 24 or 48 h. In some cases, mDCs were pretreated with varying doses of iloprost (10−9 to 10−7 mol/L) or treprostinil (10−8 to 10−6 mol/L) for 2 h and were stimulated with polyinosinic-polycytidylic acid (poly I:C; 10 µg/mL) for 6, 24 or 48 h without the washout of the PGI2 analogs. Supernatants were collected for IL-10 and TNF-α measurement. For the experiment of DC/T cell coculture, mDCs were treated with iloprost (10−9 to 10−7 mol/L) for 48 h and were washed with phosphate-buffered saline (PBS) for three times before being cocultured with T cells.
To examine the involvement of the IP receptor, E-prostanoid (EP) receptor and peroxisome proliferator-activated receptors (PPARs) in the effects of PGI2 analogs, mDCs were pretreated with the IP receptor antagonist (CAY 10449), EP1 receptor antagonist (SC19220), EP2 receptor antagonist (AH6809), EP4 receptor antagonist (GW627368X), PPAR-α antagonist (GW6741) or PPAR-γ antagonist (GW 9662) at the concentration of 10−5 to 10−6 mol/L either alone or 1 h before the treatment of the cells with iloprost or treprostinil and then were treated with or without poly I:C 2 h after iloprost or treprostinil treatment. All IP receptor, EP receptor and PPAR antagonists were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). To examine the involvement of intracellular calcium (Ca2+) in the effects of iloprost, mDCs were pretreated with the intracellular free calcium chelator BAPTA-AM (Sigma-Aldrich, St. Louis, MO, USA) at the concentration of 10−5 to 10−6 mol/L either alone or 15 min before iloprost treatment and were then treated with or without poly I:C 2 h after iloprost treatment. In some cases, mDCs were treated with forskolin, an adenyl cyclase activator, for 24 h or pretreated with forskolin for 2 h and then stimulated with poly I:C for 24 h. To investigate the cell signaling, the cells were pretreated with mitogen-activated protein kinase (MAPK)-p38 inhibitor (SB203580), MAPK-JNK (Jun NH2-terminal kinase) inhibitor (SP600125) or MAPK-ERK (extracellular signal-related kinase) inhibitor (PD98059) for 1 h and were stimulated with poly I:C for 24 h. The concentration used in experiments is according to the half maximal inhibitory concentration (IC50) of each MAPK inhibitor and previous studies (21,22). All MAPK inhibitors were purchased from Cayman Chemical Company. Supernatants were collected for IL-10 and TNF-α measurement.
Intracellular Ca2+ Measurements
Intracellular Ca2+ levels were measured using Fluo-3-acetoxymethylester (Fluo-3-AM) as our previous work (23). Briefly, human mDCs were washed with Ca2+-free PBS and then incubated with Fluo-3-AM (5 µmol/L) for 30 min. After being washed with Ca2+-free PBS, mDCs were treated with iloprost (10−7 mol/L) for 2 h and washed and resuspended in calcium-free PBS. The fluorescence intensities of Fluo-3-AM, which reflect the levels of intracellular Ca2+ level, were measured using flow cytometry.
cAMP Assay
A commercial 3′,5′-cyclic monophosphate (cAMP) enzyme immunoassay kit (Sigma-Aldrich) was used for intracellular cAMP measurement. Human mDCs (1 × 106) were incubated with or without iloprost or treprostinil (10−9 to 10−7 mol/L) for 30 min and were lysed with lysis buffer. After 10 min, the total cell lysates were centrifuged at 600g for 10 min. The supernatants were used for detecting intracellular cAMP following the manufacturer’s instruction.
Enzyme-Linked Immunosorbent Assay
The level of cytokines in the culture supernatants was determined for TNF-α, IL-10, IL-13 and interferon (IFN)-γ by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Cytosolic and Nuclear Protein Extraction
The nuclear and cytosolic fractionation techniques were used in our previously published work (20,22). Briefly, human mDCs (106) cells were pretreated with or without iloprost (10−7 mol/L) for 2 h or pretreated with or without the MAPK-p38 inhibitor SB203580 (5 µmol/L) for 1 h and were stimulated with 10 µg/mL poly I:C for 1 h. The cells were washed with iced PBS once and then resuspended. The cells were lysed in 10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 300 mmol/L sucrose, 0.5% NP-40 and proteinase inhibitor cocktail (1.0 mmol/L phenyl-methylsulfonyl fluoride, 1.0 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 µmol/L pepstatin A, leupeptin 1 µmol/L, 0.1 µmol/L aprotinin) for 3 min on ice and then centrifuged at 7,050g for 20 s. The supernatants were collected as cytosolic protein lysate. The precipitants were resuspended using 20 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 420 mmol/L NaCl, 1 mmol/L dithiothreitol, 0.2 mmol/L EDTA, 25% glycerol and proteinase inhibitor cocktail on ice for 30 min and were then centrifuged at 13,000g for 5 min. The supernatants were collected as nuclear protein lysates.
Western Blotting
After treatment for 2 h with or without iloprost (10−7 mol/L), the mDCs were stimulated with 10 µg/mL poly I:C for 1 h and then lysed with equal volumes of icecold 150-µL lysis buffer. After centrifugation at 13,000g for 15 min, equal amounts of cell lysates (20 µg) were analyzed by Western blotting with anti-p65, anti-MAPK (p38, ERK and JNK), anti-phospho-p65 (pp65) and anti-phospho-MAPK (pp38, pERK and pJNK) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). MAPK-p38 activities in cells were measured by nonradioactive MAPK-p38 assay kits (Cell Signaling Technology, Danvers, MA, USA) using the protocols recommended by the manufacturer. Activating transcription factor (ATF)2 was used as substrates for p38 MAPK assay, and phospho-ATF2 and ATF2 were analyzed by Western blotting using anti-phospho-ATF2 and anti-ATF2 antibodies (Cell Signaling Technology). Cytosolic and nuclear protein lysates were analyzed by Western blotting using anti-mixed lineage leukemia (MLL) antibody (Bethyl Laboratory, Montgomery, TX, USA), anti-WD repeat domain 5 (WDR5) antibody (Millipore-Upstate, Billerica, MA, USA), anti-α-tubulin antibody (Sigma-Aldrich) and anti-histone H3 antibody (Millipore-Upstate). Immunoreactive bands were visualized using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Sunnyvale, CA, USA).
T-Cell Stimulation Assay
Autologous CD4+ T cells were purified from PBMCs with human CD4 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Isolated CD4+ T cells (106/well) were cocultured with iloprost-treated or vehicle-treated mDCs (105/well) as described above in 24-well round-bottom plates in 1 mL/well for 5 d in the presence of anti-CD3 and anti-CD28 antibodies (eBioscience, San Diego, CA, USA). In some conditions, CD4+ T cells were cocultured with iloprost-treated mDCs in the presence of anti-IL-10 antibody (0.1 µg/mL; eBioscience). Supernatants were collected for IL-13, IFN-γ and IL-10 measurement.
Flow Cytometry Analysis
Isolated mDCs were cultured in 12-well round-bottom plates (106/1 mL/well) and were treated with iloprost (10−7 mol/L) for 2 h and then stimulated with poly I:C (10 µg/mL) for 48 h and were harvested and washed three times with PBS for direct immunofluorescence staining using fluorescein isothiocyanate-labeled monoclonal antibodies to CD11c, CD40 or CD80, and phycoerythrin-labeled monoclonal antibodies to CD86 or human leukocyte antigens (HLA)-DR. All fluorescence-conjugated monoclonal antibodies were purchased from eBio-science. The surface markers of mDCs were analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
Chromatin Immunoprecipitation (ChIP) Assay
Chromatin immunoprecipitation (ChIP) assay was performed as described in our previously published work (20,22). Briefly, 5 × 105 mDCs in each condition were treated with 1% formaldehyde for 10 min at room temperature. Lysed cells were sonicated and immunoprecipitated overnight at 4°C with anti-trimethylated H3K4 antibody (Upstate Biotechnology, Waltham, MA, USA) or rabbit anti-BSA (Sigma-Aldrich) as a control. Antibody-bound complexes were collected with a slurry of protein A (Invitrogen, Carlsbad, CA, USA) and were washed extensively, and immune complexes were eluted. DNA was extracted by phenol-chloroform after reverse cross-linking for 6 h at 65°C and after protein removal by proteinase K (200 µg/mL; Roche Diagnostics, Nutley, NJ, USA) treatment in the presence of 20 µg/mL glycogen. The DNA was finally RNase treated (40 mg/mL; Roche Diagnostics, Nutley, NJ, USA) for 30 min at 37°C and quantitated before analyses. Total DNA amount of each DNA sample was measured. Equal DNA amount of each sample was used to perform polymerase chain reaction to quantitate the amount of DNA from the promoter and enhancers regions of the TNFA gene encompassing the various TNF-α promoter regions relative to the transcription start sites (17): TNF1 (+99/-42); TNF2 (+32/−119), TNF3 (-100/−250), TNF4 (-195/−345), 1700 (-1694/−1758), +1417(+1391/+1431)and +720(+762/+799). Polymerase chain reactions were run on the ABI 7700 Taqman thermocycler (Applied Biosystems, Foster City, CA, USA). All Taqman reagents were purchased from Applied Biosystems. The relative amounts of the amplified product were normalized to the total input DNAs.
Statistical Analysis
For each experiment, three replicates were performed for each subject’s mDCs, and at least three subjects’ mDCs were used to confirm the results presented. All data are presented as the mean ± standard deviation (SD). Differences between experimental and control groups were analyzed using the Wilcoxon signed-rank test. A P value <0.05 was considered indicative of significant between-group differences.
Results
Iloprost and Treprostinil Enhanced IL-10 and Suppressed TNF-α Expression in Human mDCs
To investigate the potential effect of PGI2 analogs on the expression of cytokines in human mDCs, mDCs isolated from healthy subjects were treated with varying doses of iloprost or treprostinil, either alone or in combination with tolllike receptor (TLR)-3 agonist poly I:C. As shown in Figure 1, iloprost (10−9 to 10−7 mol/L) alone or combined with poly I:C could enhance IL-10 expression in human mDCs (Figures 1A, B). Treprostinil (10−8 and 10−7 mol/L) alone could also enhance IL-10 production in human mDCs (Figure 1C). Of interest, iloprost (10−8 to 10−7 mol/L) could suppress poly I:C-induced TNF-α expression (Figure 1D), and treprostinil at a higher concentration (10−6 mol/L) could also suppress poly I:C-induced TNF-α expression (Figure 1E) in human mDCs. However, iloprost or treprostinil alone without the presence of poly I:C had no effect on TNF-α expression (data not shown).
Iloprost and treprostinil enhanced IL-10- and poly I:C-induced IL-10 expression and suppressed poly I:C-induced TNF-α expression in human mDCs. Isolated mDCs were pretreated with iloprost or treprostinil for 24 or 48 h, or for 2 h, and then stimulated with poly I:C for 6, 24 or 48 h. Supernatants were collected for cytokine measurement. Iloprost alone (10−8 to 10−7 mol/L) (A) or in the presence of poly I:C (10−9 to 10−7 mol/L) (B) enhanced IL-10 expression in human mDCs. Treprostinil alone (10−9 to 10−7 mol/L) (C) also enhanced IL-10 expression. Iloprost (10−8 to 10−7 mol/L) (D) and treprostinil at higher concentration (10−6 mol/L) (E) suppressed poly I:C-induced TNF-α expression. Results presented are the mean ± SD of six independent experiments using mDCs from six subjects. *P < 0.05 compared with vehicle-treated cells (A, C) or poly I:C-treated cells (B, D, E). (A, B) □, 6 h; □, 24 h; ■, 48 h.
PGI2 Analogs Modulated IL-10 and TNF-α Expression in mDCs via the IP and EP Receptors but Not the PPARs
It is known that PGI2 analogs can exert their function through the IP and EP receptors (24) and are also PPAR ligands with antiinflammatory actions (25). We have previous demonstrated that iloprost and treprostinil modulate chemokine expression partly via the PPARs in human monocytes (20).To examine whether the modulatory effect of PGI2 analogs on IL-10 and TNF-α expression is mediated through the IP receptors, EP receptors or PPARs, mDCs were pretreated with the IP receptor antagonist (CAY 10449), the EP receptor antagonists (SC 19220 as EP1 receptor antagonist; AH6809 as EP2 receptor antagonist; GW627368X as EP4 receptor antagonist) or PPAR antagonists (GW6741 as PPAR-α antagonist; GW9662 as PPAR-γ antagonists) at the concentration of 10−6 to 10−5 mol/L. As shown in Figure 2, the addition of IP receptor antagonist CAY 10449 (10−6 to 10−5 mol/L) reversed iloprost-enhanced IL-10 expression (Figure 2A). The EP1 receptor antagonist SC 19220 (10−5 mol/L), but not EP2 or EP4 receptor antagonists, reversed iloprost-enhanced IL-10 expression (Figure 2B). These data suggested that iloprost enhanced IL-10 expression of mDCs through the IP and EP1 receptors. As shown in Figures 2C and D, the IP receptor antagonist (10−6 to 10−5 mol/L) and EP1, EP2 and EP4 receptor antagonists at 10−5 mol/L significantly reversed iloprost-suppressed poly I:C-induced TNF-α expression in mDCs, implicating that iloprost suppressed poly I:C-induced TNF-α expression through the IP, EP1, EP2 and EP4 receptors in mDCs. As for treprostinil, only the EP4 receptor antagonist GW627368X at the concentration of 10−5 mol/L, but not the IP, EP1 or EP2 receptor antagonists (10−5 mol/L), reversed treprostinil-enhanced IL-10 expression (Figures 3A, B). The IP receptor antagonist at the concentration of 10−5 mol/L, but not EP1, EP2 or EP4 receptor antagonist, could reverse treprostinil-suppressed poly I:C-induced TNF-α expression in mDCs (Figure 3C). Each of the IP or EP receptor antagonists alone had no effects on IL-10- or poly I:C-induced TNF-α expression (data not shown). Taken together, treprostinil enhanced IL-10 via the EP4 receptor and suppressed poly I:C-induced TNF-α expression via the IP receptor in mDCs. However, PPAR-α antagonist (GW 6741) and PPAR-γ antagonists (GW9662) did not reverse iloprost-or treprostinil-enhanced IL-10 expression (Figures 4A, B). PPAR-α and PPAR-γ antagonists also did not reverse iloprost-or treprostinil-suppressed poly I:C-induced TNF-α expression (Figures 4C, D). PPAR-α and PPAR-γ antagonists alone also had no effects on IL-10- or poly I:C-induced TNF-α expression (data not shown). These data suggested that PPARs may not be involved in the effect of iloprost and treprostinil on IL-10- and poly I:C-induced TNF-α expression in human mDCs.
Iloprost enhanced IL-10 expression and suppressed poly I:C-induced TNF-α expression in mDCs via the IP and EP receptors in human mDCs. Isolated human mDCs were pretreated with IP receptor antagonist CAY 10449 or EP receptor antagonists 1 h before treatment of iloprost. (A) CAY 10449 reversed iloprost-enhanced IL-10 expression. (B) The EP1 receptor antagonist SC19220 (10−5 mol/L), but not EP2 or EP4 receptor antagonists, also reversed iloprost-enhanced IL-10 expression. CAY 10449 (C) and EP1, EP2 and EP4 receptor antagonists (D) all significantly reversed iloprost-suppressed poly I:C-induced TNF-α expression. Results presented are the mean ± SD of six independent experiments using mDCs from six subjects. *P < 0.05 compared with iloprost-treated cells (A, B) or iloprost plus poly I:C-treated cells (C, D).
Treprostinil enhanced IL-10 expression via the EP4 receptor and suppressed poly I:C-induced TNF-α expression via the IP receptor in human mDCs. Isolated human mDCs were pretreated with IP receptor antagonist CAY 10449 or EP receptor antagonists 1 h before treatment of treprostinil. (A) CAY 10449 was unable to reverse treprostinil-enhanced IL-10 expression. (B) EP4 receptor antagonist GW627368X (10−5 mol/L), but not EP1 or EP2 receptor antagonists, reversed treprostinil-enhanced IL-10 expression. (C) The IP receptor antagonist CAY 10449, but not the EP1, EP2 or EP4 receptor antagonist, reversed treprostinil-suppressed poly I:C-induced TNF-α expression in mDCs. Result represented the mean ± SD of six independent experiments using the mDCs of six subjects. *P < 0.05 compared with treprostinil-treated cells (B) or treprostinil plus poly I:C-treated cells (C).
The modulatory effects of PGI2 analogs on IL-10 and TNF-α expression is independent of PPARs in human mDCs. To investigate the involvement of PPARs in the modulatory effect of PGI2 analogs on IL-10 and TNF-α expression in human mDCs, the cells were pretreated with PPAR-α antagonist (GW 6741) and PPAR-γ antagonists (GW9662) 1 h before the treatment of iloprost or treprostinil. Neither PPAR-α antagonist nor PPAR-γ antagonist changed the enhancing effect of iloprost (A) or treprostinil (B) on IL-10 expression, and the suppressive effect of iloprost (C) or treprostinil (D) on poly I:C-induced TNF-α expression. Results represented are the mean ± SD of three independent experiments using mDCs from three subjects.
PGI2 Analogs Modulated IL-10 and TNF-α Expression in mDCs via the cAMP Pathway, and Iloprost Also Modulated IL-10 and TNF-α Expression via the Ca2+ Pathway
IP and EP receptors are G proteincoupled receptors, and the cellular responses are based on the types of G protein. It is known that IP, EP2 or EP4 receptors activate the G protein Gs, which leads to an increase of intracellular cAMP (20,24). We next examined whether iloprost or treprostinil could elevate intracellular cAMP in human mDCs. As shown in Figures 4A and B, iloprost (10−8 to 10−7 mol/L) and treprostinil (10−7 mol/L) result in an increase of intracellular cAMP in mDCs. Next we used forskolin, an adenyl cyclase activator, to examine whether elevating cAMP could confer a similar effect in human mDCs. Forskolin also enhanced IL-10 expression (Figure 4C) and suppressed poly I:C-induced TNF-α expression (Figure 4D) in human mDCs. Taken together, these data suggested the modulatory effects of PGI2 analogs on IL-10 and TNF-α expression were through the IP/EP2/EP4-cAMP pathway.
The activation of the EP1 receptor by ligands increases the level of the intracellular Ca2+, which regulates the downstream signals (26). Because the involvement of EP1 receptor in the modulatory effects of iloprost on IL-10- and poly I:C-induced TNF-α is suggested by the results using EP1 receptor antagonist, we next investigated whether the EP1-Ca2+ pathway is involved in the modulatory effects of iloprost. We used flow cytometry to investigate the effect of iloprost on the change of intracellular Ca2+ levels. As shown in Figures 4E and F, treatment with iloprost (10−7 mol/L) enhanced the fluorescence intensity of Fluo-3-AM, and the effects could be partly reversed by EP1 receptor antagonist SC 19220 (10−5 mol/L), suggesting that iloprost increased intracellular Ca2+ levels via the EP1 receptor. BAPTA-AM, the intracellular free Ca2+ chelator, partly abrogated the modulatory effects of iloprost on IL-10-induced (Figure 5G) and poly I:C-induced TNF-α (Figure 5H) expression. Taken together, this evidence suggested that iloprost may also partly modulate IL-10- and poly I:C-induced TNF-α expression via the EP1 receptor-Ca2+ pathway.
PGI2 analogs modulate IL-10 and TNF-α expression via the IP-EP2/EP4-cAMP pathway, and iloprost also modulated IL-10 and TNF-α expression via the EP1-Ca2+ pathway in human mDCs. Isolated human mDCs were treated with iloprost or treprostinil for 24 h. Iloprost (10−8 to 10−7 mol/L) (A) and treprostinil (10−7 mol/L) (B) increased intracellular cAMP levels in human mDCs. Forskolin, an adenyl cyclase activator, enhanced IL-10 expression (C) and suppressed poly I:C-induced TNF-α expression (D). To verify the involvement of EP1-Ca2+ pathway in the modulatory effect of iloprost, cells were treated with iloprost (10−7 mol/L) for 2 h with or without pretreatment of EP1 receptor antagonist SC 19220 (10−5 mol/L) and were detached and labeled with Fluo-3-AM. The change of intracellular Ca2+ was analyzed using flow cytometry. One experiment representative of three is shown as a histogram plot graph (E). (F) Iloprost increased cytosol Ca2+ level by the measurement of the mean fluorescence intensity (MFI), and the EP1 receptor antagonist SC 19220 partly reversed the effect. BAPTA-AM, an intracellular free Ca2+ chelator, partly reversed the modulatory effects of iloprost on IL-10 (G) and poly I:C-induced TNF-α expression (H). Results represent the mean ± SD of three independent experiments using mDCs from three subjects for the cAMP assay, the flow cytometry analysis for intracellular Ca2+ and the BAPTA-AM experiment, and of six independent experiments using mDCs from six subjects for the forskolin experiment. *P < 0.05 compared with vehicle-treated cells (A, B, C, F, G) or poly I:C-treated cells (D, H). #P < 0.05 compared with iloprost-treated cells.
Iloprost Suppressed Poly I:C-Induced TNF-α Expression in Human mDCs via the MAPK-p38-ATF2 Pathway
It is known that the activation of Tolllike receptors on DCs can lead to activation of the MAPK pathways including the MAPK-p38, JNK and ERK pathways (27). It is also known that the nuclear factor (NF)-κB pathway is involved in the expression of proinflammatory cytokines in DCs (14). We next investigated whether the effects of PGI2 analogs on TNF-α expression is via these pathways. As shown in Figure 5, all three MAPK inhibitors suppressed poly I:C-induced TNF-α expression (Figure 6A), suggesting that all three MAPK pathways are involved in poly I:C-induced TNF-α expression in mDCs. Western blotting revealed that iloprost suppressed poly I:C-induced phospho-p38 and phospho-ATF2 expression in mDCs (Figures 6B, C). However, iloprost had no effect on poly I:C-induced phospho-p65, phospho-ERK or phospho-JNK expression in mDCs (data not shown). These data suggested that iloprost may suppress poly I:C-induced TNF-α expression in human mDCs via the MAPK-p38-ATF2 pathway.
Iloprost suppressed poly I:C-induced TNF-α expression in human mDCs via the MAPK-p38-ATF2 pathway. Isolated human mDCs were pretreated with MAPK-p38 inhibitor (SB203580), MAPK-JNK inhibitor (SP600125) or MAPK-ERK inhibitor (PD98059) 1 h before poly I:C stimulation. Supernatants were collected 24 h after poly I:C stimulation for TNF-α measurement. For Western blotting analysis, mDCs were pretreated with or without iloprost (10−7 mol/L) for 2 h and then stimulated with poly I:C for 1 h, and cell lysates were collected. (A) All MAPK inhibitors suppressed poly I:C-induced TNF-α expression. Iloprost suppressed poly I:C-induced phospho-p38 (B) and phospho-ATF2 (C) expression. Result of TNF-α expression represented the mean ± SD of six independent experiments using mDCs from six subjects. For Western blotting analyses, one experiment representative of three is shown. *P < 0.05 compared with poly I:C-treated cells.
Iloprost Suppressed Poly I:C-Induced TNF-α Expression via Histone Trimethylation
TNF-α expression can be regulated epigenetically via core histone modification in monocytes and macrophages (17). To investigate whether the effect of iloprost on poly I:C-induced TNF-α expression in mDCs is via epigenetic regulation, ChIP assays were performed with antibodies recognizing trimethylated H3K4 as a marker of gene activation (28). As shown in Figure 7, iloprost downregulated poly I:C-induced H3K4 trimethylation in the TNFA gene promoter region from upstream to downstream of the transcription start site (Figure 7A). Because histone trimethylation is mediated by methyltransferase, we next examine whether the downregulation of H3K4 trimethylation by iloprost is associated with the downregulation of the H3K4-specific methyltransferases and MLL and WDR5 proteins (9). Intriguingly, poly I:C-induced translocation of MLL and WDR5 protein from cytoplasm into nucleus, and iloprost, can partly reversed the translocation of MLL and WDR5 proteins from cytoplasm to nucleus by poly I:C (Figures 7B, D). In the present study, we show that iloprost inhibited the poly I:C-induced phosphorylation of MAPK-p38. Therefore, we next investigated whether MAPK-p38 signaling is involved in poly I:C-induced subcellular localization of MLL and WDR5. As shown in Figure 7C, the MAPK-p38 inhibitor SB203580 also suppressed translocation of MLL and WDR5 proteins from cytoplasm to nucleus. The evidence suggested that the inhibitory effect of iloprost on the poly I:C-induced translocation of MLL and WDR5 from cytoplasm to nucleus may be, at least partly, via the MAPK-p38 pathway. Taken together, the present study suggested that the effect of iloprost on histone H3K4 trimethylation in the TNFA gene promoter region may play an important role in regulating the expression of TNF-α in human mDCs.
Iloprost suppressed poly I:C-induced TNF-α expression in mDCs via histone H3K4 trimethylation in the TNFA gene promoter region. Isolated mDCs were pretreated with iloprost for 2 h and then stimulated with poly I:C for 1 h and were used for ChIP assay using anti-H3K4 antibodies. The cells were also used for cytosolic and nuclear protein analysis by Western blotting using anti-MLL and anti-WDR5 antibodies. The nuclear and cytosolic fractionation technique was verified using anti-α-tubulin and anti-histone H3 antibodies. (A) Iloprost downregulated poly I:C-induced H3K4 trimethylation in the TNFA gene promoter region. (B) Iloprost suppressed poly I:C-induced translocation of cytosolic H3K4-specific methyltransferases MLL and WDR5 proteins into nucleus. The nuclear fraction is lack of α-tubulin, and the cytosolic fraction is lack of H3. To investigate whether MAPK-p38 signaling is responsible for the translocation of MLL and WDR5 from cytoplasm to nucleus, mDCs were pretreated with MAPK-p38 inhibitor SB203580 for 2 h and then stimulated with poly I:C for 1 h. (C) SB203580 suppressed poly I:C-induced translocation of MLL and WDR5 from cytoplasm to nucleus. (D) Densitometry analysis of the Western blot data shown in (B). Results represented are the mean ± SD of three independent experiments using mDCs from three subjects for the ChIP assay and densitometry analysis. For Western blotting analyses, one experiment representative of three is shown. *P ≤ 0.05 compared with poly I:C-treated cells (A and D). OD, optical density. A: □, Control; ■, poly I:C; ■, iloprost + poly I:C.
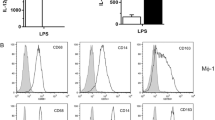
Iloprost Had No Effect of CD86, CD80, CD40 and HLA-DR Expression on mDCs
To examine the effect of iloprost on poly I:C-induced mDC maturation, the expression of costimulatory molecules including CD86, CD80, CD40 and HLA-DR was investigated by flow cytometry. However, during the culture time period, there was no significant difference in the expression levels of DC maturation markers, including CD86, CD80, CD40 and HLA-DR, as judged by flow cytometry (data not shown).
Iloprost-Treated mDCs Suppressed IL-13, IFN-γ and IL-10 Production in CD4+ T Cells
To investigate the effect of iloprost on the ability of mDCs to stimulate T-cell response, human mDCs were treated with varying doses of iloprost (10−9 to 10−7 mol/L) and were cocultured with autologous CD4+ T cells at a ratio of 1:10 (105 mDCs/106 CD4+ T cells) in the presence of anti-CD3 and anti-CD28. In some cases, IL-10 neutralizing antibody was used to verify whether the effects of iloprost on IL-13/IFN-γ production by T cells is through IL-10 regulation. As shown in Figure 8, IL-13 (Figure 8A), IFN-γ (Figure 8B) and IL-10 (Figure 8C) production by CD4+ T cells was significantly suppressed by iloprost-treated mDCs. The presence of IL-10-neutralizing antibody did not change the effect of iloprost-treated mDCs on the IL-13 and IFN-γ production by T cells (Figure 8D). These data suggested iloprost-treated mDCs may attenuate the ability of mDC to stimulate T-cell response, and the effects were not indirectly mediated by IL-10.
Iloprost-treated mDCs suppressed IL-13, IFN-γ and IL-10 production by CD4+ T cells. Autologous CD4+ T cells were isolated and then cocultured with vehicle-treated or iloprost-treated mDCs (105 mDCs/106 T cells) for 5 d in the presence of anti-CD3 and anti-CD28 antibodies with or without the addition of IL-10 neutralizing antibody. Supernatants were collected for IL-13, IFN-γ and IL-10 measurement. Iloprost-treated mDCs significantly suppressed IL-13 (A), IFN-γ (B) and IL-10 production (C) by CD4+ T cells. (D) The addition of IL-10 neutralizing antibody did not change the effect of iloprost-treated mDCs on IL-13 and IFN-γ production by T cells. Results represent the mean ± SD of six independent experiments using mDCs from six subjects in the experiments without the addition of IL-10 neutralizing antibody and of three independent experiments using mDCs from three subjects in the experiments with the addition of IL-10 neutralizing antibody. *P ≤ 0.05 compared with the group of vesicle-treated mDC/T cell coculture. §P ≤ 0.05 compared with the group of iloprost-treated mDC/T-cell coculture without the addition of IL-10 neutralizing antibody. (D) □, Control; ■, iloprost (10−7 mol/L); ■, iloprost (10−7 mol/L) + anti-IL-10.
Discussion
DCs are the chief orchestrators of immune responses. The crucial task of mDCs is the continuous surveillance of antigen-exposed sites throughout the body and the initiation of primary T-cell responses including T-cell polarization into Th1 and Th2 cells by secreting cytokines and expressing costimulatory molecules after activation (17,29). DCs, particular in mDCs, have a specific and important role in pathogenesis of human asthma (11–13). Recently, PGI2 is regarded as a potential treatment of asthma by their antiinflammatory effect in vitro (14,30) and in animal model (15). The antiinflammatory effect of PGI2 analogs by altering the function of DCs has been revealed by our previous work using human plasmacytoid DCs (16) and by the work of Muller et al. (31) using human monocyte-derived DCs (31). However, the effect of PGI2 analogs on human mDCs is not elucidated. In the present study, we demonstrated, for the first time, the effect of PGI2 analogs (iloprost and treprostinil) on human mDCs and found that although iloprost had no effect on costimulatory molecules expression, iloprost and treprostinil could enhance IL-10 and suppress poly I:C-induced TNF-α expression in mDCs. In addition, iloprost could suppress the ability of mDCs to stimulate Th1 (IFN-γ) and Th2 (IL-13) response. These all implicate the potential role of PGI2 analogs in treating asthma by altering the function of mDCs.
Prostaglandins are derived from arachidonic acid by stepwise conversion and are important endogenous inflammatory mediators, controlling immune stimulation and inflammation by the effects of prostaglandins on cytokine production (32). IP receptor activation by PGI2 or its analogs can result in vasodilation and antithrombotic and antiinflammatory effects (14,33). PGI2 analogs are available in different formulations and are potent ligands with different binding affinities for the various prostanoid receptors. Ioprost was reported with affinity to the IP, EP1, EP2, EP3 and EP4 receptors, and treprostinil was reported with affinity to the IP and EP2 receptors (34–36). The physiological activities of the analogs depend on the receptor they activate, and these effects can be receptor-specific. For example, while treprostinil is clearly a potent IP receptor agonist (37), the effect of treprostinil on inhibiting phagocytosis, bacterial killing, and cytokine generation in the alveolar macrophage is via the EP2 receptor but not IP receptor (24). In the present study, we demonstrate that the modulatory effects of PGI2 analogs on IL-10 and TNF-α expression in human mDCs involved different types of receptors. To enhance IL-10 expression, iloprost acted through the IP and EP1 receptor, whereas treprostinil acted through the EP4 receptor only. To suppress poly I:C-induced TNF-α expression, iloprost acted through IP, EP1, EP2 and EP4 receptors, while treprostinil acted through IP receptor only. The different involvement of IP/EP receptors of iloprost and treprostinil may partly explain their different potency in modulating cytokine expression. Although PGI2 analogs are PPAR ligands with antiinflammatory actions (25) and our previous work demonstrated that iloprost and treprostinil modulate chemokines expression partly via PPARs in human monocytes (20), the present study revealed that the PPARs were not involved in the modulatory effect of PGI2 analogs on IL-10 and TNF-α expression in human mDCs.
In the present study, we also demonstrated the responsible signal transduction pathway that was activated by the prostanoid receptor for the modulatory effect of PGI2 analogs on IL-10- and poly I:C-induced TNF-α expression in human mDCs. It has been suggested that the signaling followed by the activation of prostanoid receptor depends on the types of coupled G protein. The activation of the G protein may vary from ligands and the ligand concentration and finally evokes different cellular responses (34). The IP and EP2/EP4 receptors are coupled to Gs protein and activates adenyl cyclase, which results in a burst of intracellular cAMP, whereas EP1 receptors are coupled to the Gq protein and mediate the increase of intracellular Ca2+ (38). In the present study, we demonstrated that iloprost, and treprostinil at higher concentrations, increased intracellular cAMP levels. Forskolin, the adenyl cylase activator, modulated similar effects on IL-10 and TNF-α expression. These results suggested the enhancing effect on IL-10 may be via the IP-cAMP pathway by iloprost and via the EP4-cAMP pathway by treprostinil, and the suppressive effect on poly I:C-induced TNF-α may be via the IP-EP2/EP4-cAMP pathway by iloprost and via the IP-cAMP by treprostinil. Interestingly, the potency of forskolin on enhancing IL-10 and suppressing poly I:C-induced TNF-α expression is quite different. Compared to the nearly complete reversing effect on iloprost-enhanced IL-10 expression, the IP receptor antagonist CAY 10449 restored the suppressive effect of iloprost on poly I:C-induced TNF-α expression to a less extent. These observations implicate there may be a cAMP-independent pathway that mediated the suppressive effect of iloprost on poly I:C-induced TNF-α expression. We found that iloprost increased intracellular Ca2+ levels via the EP1 receptor, and the intracellular Ca2+ chelator BAPTA-AM abrogated the modulatory effect of iloprost on IL-10- and poly I:C-induced TNF-α expression. The evidence indicates that in addition to IP-EP-cAMP pathways, iloprost can also modulate IL-10- and poly I:C-induced TNF-α expression via the EP1-Ca2+ pathway. The higher potency on increasing intracellular cAMP level and the activation of an additional pathway may partly explain the higher potency of iloprost in modulating cytokine expression compared to that of treprostinil. Our results may offer an experimental basis for investigating the different functions and efficacy between various PGI2 analogs for clinical application.
The MAPK pathways are fundamental regulators for chemoattraction, inflammatory mediator production and activation in immune cells in response to stimulation by TLR agonists (27). In asthmatic patients, the level of phosphorylation of p38 and ERK is positively correlated with disease severity (39). In the present study, we used the MAPK-p38 inhibitor (SB203580) and Western blotting to verify that iloprost may suppress poly I:C-induced TNF-α expression via, at least partly, the MAPK-p38-ATF2 pathway, providing further understanding for the intracellular mechanism of PGI2 analogs on cytokine modulation.
In the present study, we furthermore provided an important novel finding for the epigenetic regulation of iloprost on TNF-α expression in human mDCs. Histone and DNA modifications are associated with gene transcription. For example, acetylation of core histone by histone acetyltransferase allows the chromatin structure to transform from the resting closed conformation to an activated open form, leading to gene expression. Asthma is associated with overexpression of inflammatory genes in the airway. It has been shown that the activity of histone acetyltransferase is increased in bronchial biopsy and alveolar macrophages isolated from asthmatic patients (40), and the proinflammatory cytokine, TNF-α, can be regulated epigenetically with histone acetylation in monocytes and macrophages (17). In addition to histone acetylation, histone methylation is also associated with either positive or negative transcriptional states, depending on the sites of modification. In our recently published work, we demonstrated that iloprost can modulate Th1- and Th2-related chemokine expression via histone acetylation and trimethylation in human monocytes (20). In the present study, we revealed, for the first time in human mDCs, that poly I:C could induce histone H3K4 trimethylation in the TNFA gene promoter region, and iloprost could downregulate poly I:C-induced H3K4 trimethylation in the TNFA gene promoter region. We also verified that the suppressive effect of iloprost on poly I:C-induced H3K4 trimethylation was produced by inhibiting the poly I:C-induced translocation of H3K4-specific methyltransferases MLL and WDR5 proteins from cytoplasm into nucleus. To our best knowledge, this novel mechanism by which iloprost modulates TNF-α expression in the present study is reported in the literature. Interestingly, phosphorylation signaling has been shown to play an essential role in regulating the function and interaction between proteins, maintaining the stability and participating in the localization of the protein. Recently, it was suggested that the phosphorylation modification on Thr-912 residue of MLL protein controls its subcellular localization and is required for mitotic entry (41). In the present study, we showed that iloprost suppressed poly I:C-induced phosphorylation of MAPK-p38, and by using the MAPK-p38 inhibitor SB203580, we also showed that the poly I:C-induced translocation of MLL and WDR5 proteins from cytoplasm to nucleus was MAPK-p38 dependent. These results suggest that iloprost may suppress poly I:C-induced translocation of MLL and WDR5 proteins via, at least partly, the MAPK-p38 pathway. Taken together, our findings suggest the importance of epigenetic regulation by which PGI2 analogs exert their antiinflammatory functions.
Conclusion
In conclusion, the present study provided the evidence for the effects of PGI2 analogs on human mDCs. Our study suggested that PGI2 analogs may induce tolerogenic function of human mDCs by modulating cytokine production (enhancing antiinflammatory cytokine IL-10 and suppressing proinflammatory cytokine TNF-α) and by inhibiting the ability of mDCs for T-cell stimulation. The suppressive effect of iloprost on TNF-α expression was via the IP/EP2/EP4-cAMP and EP1 receptor-Ca2+ pathway, the MAPK-p38-ATF2 pathway and epigenetic regulation by histone modification with downregulation of H3K4 trimethylation via inhibiting the translocation of H3K4-specific methyltransferases MLL and WDR5 proteins. Because of the key roles of mDCs in pathogenesis of human asthma, our results supported current evidence for the potentiality of PGI2 analogs as asthma treatment.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Barnes PJ, Chung KF, Page CP. (1998) Inflammatory mediators of asthma: an update. Pharmacol. Rev. 50:515–96.
Broide DH, et al. (1992) Cytokines in symptomatic asthma airways. J. Allergy Clin. Immunol. 89:958–67.
Brightling C, Berry M, Amrani Y. (2008) Targeting TNF-alpha: a novel therapeutic approach for asthma. J. Allergy Clin. Immunol. 121:5–10.
Berry MA, et al. (2006) Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 354:697–708.
Hawrylowicz CM, O’Garra A. (2005) Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5:271–83.
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765.
Grunig G, et al. (1997) Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185:1089–99.
John M, et al. (1998) Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 157:256–62.
Lommatzsch M, et al. (2007) Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur. Respir. J. 30:878–86.
van Rijt LS, et al. (2005) In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981–91.
Bratke K, et al. (2007) Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax. 62:168–75.
Schaumann F, et al. (2008) Endotoxin augments myeloid dendritic cell influx into the airways in patients with allergic asthma. Am. J. Respir. Crit. Care Med. 177:1307–13.
Koya T, et al. (2006) Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am. J. Respir. Crit. Care Med. 173:42–55.
Zhou W, et al. (2007) Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J. Immunol. 178:702–10.
Idzko M, et al. (2007) Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J. Clin. Invest. 117:464–72.
Hung CH, et al. (2009) Regulation of cytokine expression in human plasmacytoid dendritic cells by prostaglandin I2 analogues. Eur. Respir. J. 33:405–10.
Lee JY, Kim NA, Sanford A, Sullivan KE. (2003) Histone acetylation and chromatin conformation are regulated separately at the TNF-alpha promoter in monocytes and macrophages. J. Leukoc. Biol. 73:862–71.
Ito K, et al. (2002) Expression and activity of histone deacetylases in human asthmatic airways. Am. J. Respir. Crit. Care Med. 166:392–6.
Cosio BG, et al. (2004) Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am. J. Respir. Crit. Care Med. 170:141–7.
Kuo CH, et al. (2011) Effects of PGI(2) analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation. J. Mol. Med. 89:29–41.
Hung CH, et al. (2007) Suppressive effects of ketotifen on Th1- and Th2-related chemokines of monocytes. Pediatr. Allergy Immunol. 18:378–84.
Hung CH, et al. (2010) Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ. Health Perspect. 118:67–72.
Huang CH, et al. (2010) The natural flavonoid apigenin suppresses Th1- and Th2-related chemokine production by human monocyte THP-1 cells through mitogen-activated protein kinase pathways. J. Med. Food. 13:391–8.
Aronoff DM, et al. (2007) Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J. Immunol. 178:1628–34.
Straus DS, Glass CK. (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28:551–8.
Sugimoto Y, Narumiya S. (2007) Prostaglandin Ereceptors. J. Biol. Chem. 282:11613–7.
Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. (2001) A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 166:3837–45.
Berger SL. (2007) The complex language of chromatin regulation during transcription. Nature. 447:407–12.
Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. (1999) T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 20:561–7.
Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. (2007) Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J. Immunol. 179:6193–203.
Muller T, et al. (2010) Iloprost has potent anti-inflammatory properties on human monocyte-derived dendritic cells. Clin. Exp. Allergy. 40: 1214–21.
Helliwell RJ, Adams LF, Mitchell MD. (2004) Prostaglandin synthases: recent developments and a novel hypothesis. Prostaglandins Leukot. Essent. Fatty Acids. 70:101–13.
Hata AN, Breyer RM. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103:147–66.
Narumiya S, Sugimoto Y, Ushikubi F. (1999) Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193–226.
Lai YJ, et al. (2008) Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 178:188–96.
Mubarak KK. (2010) A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir. Med. 104:9–21.
Clapp LH, et al. (2002) Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am. J. Respir. Cell Mol. Biol. 26:194–201.
Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. (2004) Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 36:1187–205.
Liu W, et al. (2008) Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J. Allergy Clin. Immunol. 121:893–902.
Barnes PJ, Adcock IM, Ito K. (2005) Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur. Respir. J. 25:552–63.
Liu J, Wang XN, Cheng F, Liou YC, Deng LW. (2010) Phosphorylation of mixed lineage leukemia 5 by CDC2 affects its cellular distribution and is required for mitotic entry. J. Biol. Chem. 285:20904–14.
Acknowledgments
This study was supported by a grant from the Center of Excellence Environmental Medicine Kaohsiung Medical University Research Foundation (KMU-EM-98-4), the National Science Council (NSC 99-2314-B-37-014-MY3) and the Kaohsiung Medical University Hospital (KMUH-96-6G23, KMUH-97-7G51, KMUH-98-8G09 and KMUH99-9I08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Kuo, CH., Lin, CH., Yang, SN. et al. Effect of Prostaglandin I2 Analogs on Cytokine Expression in Human Myeloid Dendritic Cells via Epigenetic Regulation. Mol Med 18, 433–444 (2012). https://doi.org/10.2119/molmed.2011.00193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2011.00193