Abstract
Milk fat globule-EGF factor 8 (MFG-E8) has been shown to play an important role in maintaining the integrity of the intestinal mucosa and to accelerate healing of the mucosa in septic mice. Herein, we (a) analyzed the expression of MFG-E8 in the gut of wild-type (WT) C57BL/6 (MFG-E8+/+) mice with and without dextran sulfate sodium (DSS)-induced colitis, (b) characterized the pathological changes in intestinal mucosa of MFG-E8+/+ and MFG-E8-/- mice with DSS-induced colitis and (c) examined the therapeutic role of MFG-E8 in inflammatory bowel disease by using DSS-induced colitis model. Our data documented that there was an increase in colonic and rectal MFG-E8 expression in MFG-E8+/+ mice during the development of DSS colitis. MFG-E8 levels in both tissues decreased to below baseline during the recovery phase in mice with colitis. Changes in MFG-E8 gene expression correlated to the levels of inflammatory response and crypt-epithelial injury in both colonic and rectal mucosa in MFG-E8+/+ mice. MFG-E8-/- mice developed more severe crypt-epithelial injury than MFG-E8+/+ mice during exposure to DSS with delayed healing of intestinal epithelium during the recovery phase of DSS colitis. Administration of MFG-E8 during the recovery phase ameliorated colitis and promoted mucosal repair in both MFG-E8-/- and MFG-E8+/+ mice, indicating that lack of MFG-E8 causes increased susceptibility to colitis and delayed mucosal healing. These data suggest that MGF-E8 is an essential protective factor for gut epithelial homeostasis, and exogenous administration of MFG-E8 may represent a novel therapeutic target in inflammatory bowel disease.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a term used to describe inflammatory conditions of the large and small intestine including Crohn’s disease and ulcerative colitis. The etiology of IBD has not been elucidated completely as yet. The pathogenesis is being unraveled gradually and seems to be the result of a combination of genetic, environmental and immunological factors in which an uncontrolled exaggerated immune response within the intestinal lumen leads to inflammation in genetically predisposed individuals. IBD could be considered to be an imbalance between proinflammatory and antiinflammatory mediators in the bowel mucosa (1). Dysfunctions of the intestinal immune system and cross-reactivity against host epithelial cells have been implicated as major inflammatory mechanisms in IBD (2). A number of peptides expressed in the intestine including ghrelin, galanin, melatonin, vasoactive intestinal peptide and adrenomedullin have been shown to have an antiinflammatory effect in IBD research models (3–13).
Milk fat globule-EGF factor 8 (MFG-E8) is a glycoprotein that corresponds to the human protein lactadherin (BA46), which is known to be a marker of breast carcinomas and a major component of milk fat globules (14). MFG-E8 is an important milk mucin-associated defense component that inhibits enteric pathogen binding and infectivity (15). MFG-E8 has been shown to be present in the gut and expressed in the murine intestinal lamina propria macrophages (16–18). MFG-E8 reduces levels of proinflammatory cytokines in the inflamed colonic mucosa of dextran sodium sulfate (DSS)-treated mice (19). MFG-E8-mediated antiinflammatory effects are generated by NF-kB inhibition via the modulation of αvβ3 integrin signaling (19). MFG-E8 plays an important role in maintaining the integrity of the intestinal mucosa and accelerates healing of the mucosa in septic mice (18).
In the present study, we further examined whether MFG-E8/lactadherin is a critical peptide in the pathogenesis of IBD and if it plays a protective role. We also explored the therapeutic role of MFG-E8 in experimental colitis.
Materials and Methods
Animals
C57BL/6 wild-type (WT) mice (male, 6–10-wks-old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). MFG-E8/SED1-knockout mice were bred in our laboratory according to the methods described previously (18,20). Mice were backcrossed to C57BL/6 for 10 generations, so they were considered to be fully congenic with the C57BL/6 background. WT C57BL/6 mice were used as controls. Animals were housed in a specific pathogen-free animal facility at the Children’s Memorial Research Center (Chicago, IL, USA). All animal experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and Use Committee of the Children’s Memorial Hospital.
Colitis Induction and Administration of MFG-E8 to Mice with Established Colitis
Colitis was induced in the WT C57BL/6 mice and the MFG-E8 knockout mice by using 3.5% DSS added to drinking bottles for 1 wk. Control mice were fed only regular drinking water. The acute colitis was followed by a recovery phase by changing the drinking water containing DSS to distilled water on day 7. Mice were euthanized with CO2 inhalation at appropriate time points designed. During experiments, the mice were weighed and stools were tested for occult blood every day. The clinical colitis score was calculated by using a modified scoring system by using weight loss, hemoccult status and presence of diarrhea. In some experiments, we administrated recombinant MFG-E8 (20 ng/kg, intraperitoneally [i.p.]) to mice twice a day for 9 d during the recovery phase of colitis. Mice in control groups were given saline instead.
Histology Protocol
Animals were euthanized by carbon dioxide inhalation on days 0, 3, 7, 10 and 16 counted from day of onset of DSS feeds. Intestinal tissues were harvested immediately and fixed in 10% buffered formalin overnight and processed for routine histology. Sections were stained with hematoxylin and eosin (H&E). The histological examination was performed in a blinded manner by using a scoring system modified from a method described by Krieglstein et al. (21). Briefly, three independent parameters were measured: severity of inflammation (0 to 3: none, slight, moderate, severe), depth of injury (0 to 3: none, mucosal, mucosal and submucosal, transmural), and crypt damage (0 to 4: none, basal one-third damaged, basal two-thirds damaged, only surface epithelium intact, entire crypt and epithelium lost). The score of each parameter was multiplied by a factor reflecting the percentage of tissue involvement (×1, 0% to 25%; ×2, 26% to 50%; ×3, 51% to 75%; ×4, 76% to 100%). The final scores of severity of inflammation and depth of injury were added to a sum which was defined as inflammatory injury score. In addition, the final score of crypt damage was defined as crypt-epithelial injury score. The maximum possible inflammatory injury score and crypt-epithelial injury score are 24 and 16, respectively.
Protein Extraction, Western Blot
We used our standard protocol for isolation of total protein from intestinal tissues and immunoblotting (22). Rabbit polyclonal antibody against murine MFG-E8 (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), was used to detect MFG-E8.
RNA Extraction
Total RNA from the colonic and rectal tissues was extracted by using the RNeasy kit (QIAGEN, Valencia, CA, USA) according to the protocol of the manufacturer. RNA concentration was determined by optical densitometry at 260 with Smart Spec plus spectropho-meter (Bio-Rad, Hercules, CA, USA).
cDNA Synthesis from Total RNA by Reverse Transcription
cDNA was synthesized by using iScript cDNA synthesis kit (Bio-Rad) according to the protocol provided by the manufacturer. Briefly, 0.7 μg of RNA from each tissue sample was added to 25 μL of reaction mixture containing dNTP mix, 1 × random hexamers primer, 1 × reaction buffer, 1 μL MMLV-erived iScript reverse transcriptase which was preblended with RNAse inhibitor. The reaction was run at 25°C for 5 min, 42°C for 30 min and stopped by incubation at 85°C for 5 min. The resulting cDNA was used for the following quantitative real-time polymerase chain reaction (PCR).
Quantitative Real-Time PCR
QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA, USA) was used for the study. In brief, mastermix (73 μL) containing 0.4 nmol/L primers and 1 × SYBR Green PCR Universal Mastermix (QIAGEN) was added to 2 μL cDNA before aliquoting in triplicate to a 96-well microtiter plate (25 μL/well). The cDNA was amplified by using a Fast 7500 realtime PCR system (AB Applied Biosystems, Foster City, CA, USA) under the following conditions: 50°C for 5 min, 95°C for 10 min, and then 40 cycles of amplification (95°C for 15 sec and 60°C for 1 min). All PCR reactions were performed in 96-well plate by using a final volume of 25 μL. The cycle at which each sample crossed a fluorescence threshold, CT (at 0.1–0.2 fluorescence units), was determined. The triplicate values for each cDNA were averaged. Sequences for murine forward (F) and reverse (R) primers for real-time PCR were the following: for 18S rRNAF, 5′-TGCCCTATCA ACTTTCGATG-3′; for 18S rRNAR, 5′-GATGTGGTAGCCGTTTCTCA-3′; for MFG-E8F, 5′-ATCTACTGCCTCTGC CCTGA-3′; and for MFG-E8R, 5′-CCAGA CATTTGGCATCATTG-3′. Fold changes in expression levels of MFG-E8 mRNA in different tissues and different postnatal stages were calculated by using the 2-AACT method by using 18S rRNA as the internal reference (23). The AACT value is defined as the CT difference between the normalized amount of sample and the normalized amount of calibrator.
Statistical Analysis
Data were expressed as means ± SEM analysis of variance and one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test were used to assess the significance of differences; P < 0.05 was considered significant.
All supplementary materials are available online at https://doi.org/www.molmed.org.
Results
Clinical Course of DSS Colitis in WT and MFG-E8 Deficient mice
Exposure to DSS resulted in acute colitis in both WT and MFG-E8 deficient mice. The typical clinical symptoms included diarrhea, hematochezia and weight loss. By assessing the clinical colitis score, we revealed that DSS-treated mice became worse on day 7 but started improving once DSS feeds were stopped in both strains (Figure 1). Mice continued to have some symptoms of colitis by day 16. There is no significant difference in comparison of WT and MFG-E8 deficient mice to their clinical colitis scores.
Clinical colitis scores of WT and MFG-E8 deficient mice. WT C57BL/6J mice and MFG-E8 deficient mice (male, 6–10-wks-old) were subjected to feeding with drinking water containing 3.5% DSS for 7 d. They were given distilled water at day 7 after colitis induction until the end of experiments. All animals were monitored for clinical colitis scores as described in Materials and Methods on a daily basis. Results are expressed as mean ± SEM, n = 5. KO, knockout; ND, not detected.
Histological Changes in Colonic and Rectal Mucosa Due to DSS-Induced Colitis in WT and MFG-E8 Deficient Mice
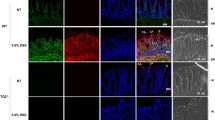
Next, we examined colon and rectum tissues from mice in each group during the DSS treatment phase through assessing severity and depth of inflammation as well as crypt-epithelial damage under a microscope. We revealed that there were no histological differences in the colon and rectum of naive WT and MFG-E8 knockout mice (data not shown). In the DSS treatment period, both colonic and rectal mucosa developed inflammation in WT and MFG-E8 knockout mice (Figure 2 and Supplementary Figure 1). We did not find any marked differences in the score of inflammation on comparing MFG-E8 knockout and WT mice (Figure 2A, C). However, MFG-E8-null mice displayed more profound crypt-epithelial injury than WT mice in both colonic and rectal mucosa during the DSS exposure phase (Figure 2B, D; and Supplementary Figure 1).
Histological grading of colonic and rectal tissue injury in DSS-treated WT and MFG-E8 deficient mice. WT C57BL/6J mice and MFG-E8 deficient mice (male, 6–10-wks-old) were subjected to DSS treatment as described in the Figure 1 legend. They were euthanized at the time points as indicated. Colonic and rectal tissues were processed for routine histology and stained with H&E. Slides were examined by using the score system as described in Materials and Methods in a doubleblind fashion. (A) Colonic inflammatory response. (B) Cryptepithelial injury in colon. (C) Rectal inflammatory response. (D) Crypt-epithelial injury in rectum. Results are expressed as mean ± SEM, n = 5. *, P < 0.05 MFG-E8 knockout versus WT mice; **, P < 0.01 MFG-E8 knockout versus WT mice. KO, MFG-E8 knockout; ND, not detected.
Furthermore, we analyzed histological alteration of colonic and rectal mucosa during the water recovery phase in DSS-treated WT and MFG-E8 knockout mice. It was revealed that mucosa of colon and rectum in DSS-treated WT and MFG-E8 knockout mice exhibited evidence of healing such as regenerating glands, decreased mucosal inflammation and ulcerations. In the colon, repair of crypt-epithelial injury but not recovery of inflammatory response was revealed to be delayed markedly in MFG-E8-null mice compared with WT mice (see Figure 2A, B; and Supplementary Figure 1). Similar to the colon, healing of mucosa in rectum was delayed in MFG-E8 knockout mice (see Figure 2C, D; and Supplementary Figure 1).
Altered Expression of MFG-E8 in DSS-Induced Acute Colitis
Furthermore, by using DSS-induced colitis model, we examined MFG-E8 expression profiles in colonic and rectal tissues at various time points in WT C57BL/6 mice with quantitative realtime (RT)-PCR and Western blots. It was revealed that expression of MFG-E8 mRNA and protein were increased significantly (P < 0.05) in inflamed colons in the early phase of the DSS treatment period (Figure 3A, B), while it was down-regulated in the late phase of the DSS treatment period and throughout the water recovery phase. MFG-E8 mRNA and protein expression in inflamed rectums was increased significantly (P < 0.05) during the DSS treatment phase while it was downregulated on during the water recovery phase (Figure 3C, D).
MFG-E8 expression profiles in colonic and rectal tissues at various time points in WT C57BL/6 mice determined with quantitative real-time RT-PCR and Western blots. WT C57BL/6J mice (male, 6–10-wks-old) were subjected to DSS treatment as described in the Figure 1 legend. They were euthanized at the time points as indicated. The expression of MFG-E8 in the colon and rectum was examined with quantitative real-time PCR and Western blotting as described in Materials and Methods. (A) MFG-E8 mRNA expression profile in colon during DSS-induced colitis. (B) MFG-E8 protein levels in colon during DSS-in-duced colitis. Top panel: representative autoradiographs of an immunoblot. Bottom panel: densitometric analysis of immunoblot data. (C) MFG-E8 mRNA expression profile in rectum during DSS-induced colitis. (D) MFG-E8 protein levels in rectum during DSS-in-duced colitis. Top panel: representative autoradiographs of an immunoblot. Bottom panel: densitometric analysis of immunoblot data. Results are expressed as mean ± SEM. n = 5 in each time point. *, P < 0.05 versus 0 d; **, P < 0.01 versus 0 d.
Administration of MFG-E8 during the Recovery Phase of Colitis Attenuates Inflammation and Enhances Epithelial Repair
In the next set of experiments, we examined whether treatment with MFG-E8 rescued MFG-E8-deficient mice from DSS-induced colitis and ameliorated DSS-induced colitis in WT mice. The mice were subjected to induction of colitis by administration of DSS in the drinking water for 7 d (colitic phase). Later these animals were switched to plain drinking water for 9 additional days (recovery phase). One group of mice started intraperitoneal (i.p.) injection with recombinant MFG-E8 (20 μ/kg) twice a day for 9 d that was started during the recovery phase, whereas another group of mice was given normal saline instead. At the end of experiments, mice were euthanized. Entire colonic and rectal tissues were processed for H&E staining followed by examination under a microscope. Sections from MFG-E8 knockout mice given DSS and not receiving recombinant MFG-E8 during the recovery phase still exhibited marked infiltration of neutrophils throughout the mucosa and submucosa, crypt loss, and epithelial ulceration in some areas in colon and rectum at the end of the 9-day recovery period (Figure 4A, left panel). In contrast, MFG-E8 knockout mice that received MFG-E8 showed decrease in colitis in colon and rectum (Figure 4A, right panel). Microscopic scores for inflammation and cryptepithelial injury were significantly lower in the DSS + MFG-E8 than in the DSS + saline group (Figure 4B, C), indicating the critical role of MFG-E8 in facilitation of mucosal repair during the recovery phase of DSS colitis. Similarly, WT mice treated with MFG-E8 during the recovery phase also displayed less severe neutrophil infiltration, crypt damage and epithelial ulceration compared with saline-treated group (Figure 4D). Semiquantitative light microscopic analysis further revealed that the severity of colitis (assessed by inflammatory injury and crypt loss) in DSS- + MFG-E8-treated WT mice at the end of the 9-day recovery period was significantly lower than that in DSS- + saline-treated WT mice (Figure 4E, F). Taken together, the data demonstrated the therapeutic efficacy of MFG-E8 in colitis.
Histological evaluation of MFG-E8 knockout and WT mice treated with MFG-E8 during the recovery phase. MFG-E8 knockout and WT mice (male, 6–10-wks-old) have ad libitum access to drinking water containing 3.5% DSS for 7 d followed by 9 d of plain drinking water (recovery). They were treated with MFG-E8 (20 μg/kg, i.p.) or equivalent volume of saline twice a day during the recovery phase. At the end of experiments, intestinal tissues were processed for histological examination as described in Figure 2. (A) Representative H&E-stained colon (top panel) and rectum (bottom panel) sections of saline- or MFG-E8-treated knockout mice (left and right panels, respectively). Original magnification 10×. (B) Histologic score of severity of inflammatory response in saline- and MFG-E8-treated knockout mice. (C) Histologic score of severity of crypt-epithelial injury in saline-and MFG-E8-treated knockout mice. (D) Representative H&E-stained colon (top panel) and rectum (bottom panel) sections of saline- or MFG-E8-treated WT mice (left and right panels, respectively). Original magnification 10×. (E) Histologic score of severity of inflammatory response in saline- and MFG-E8-treated WT mice. (F) Histologic score of severity of crypt-epithelial injury in saline- and MFG-E8-treated WT mice. Results are expressed as mean ± SEM, n = 6. *, P < 0.05 versus saline group; **, P < 0.01 versus saline group.
Discussion
MFG-E8 is being investigated currently for its cytoprotective role in many tissues including the intestinal mucosa. It has been shown that MFG-E8 is present in the gut and expressed in the murine intestinal lamina propria macrophages (16–18). Previously, we showed that MFG-E8 promotes migration of intestinal epithelial cells through a PKCɛ-dependent mechanism (18). It binds to phosphatidylserine and triggers reorientation of the actin cytoskeleton in intestinal epithelial cells at the wound edge. Aziz et al. demonstrated that pretreatment with MFG-E8 attenuates intestinal inflammation in experimental colitis by modulating osteopontindependent αvβ3 integrin signaling (19). However, the therapeutic efficacy of MFG-E8 in colitis remains unknown. In the present study, we further examined the role of MFG-E8 in DSS-induced colitis by using MFG-E8 deficient mice. We found that MFG-E8 knockout mice develop more severe crypt-epithelial injury during the onset of DSS-induced acute colitis. Interestingly, we did not reveal significant difference in intestinal inflammatory response between WT and MFG-E8 knockout mice in the colitic phase. During the recovery phase of DSS colitis, MFG-E8 knockout mice showed a delayed healing of damaged intestinal epithelium compared with WT mice. Rescue therapy with MFG-E8 during the recovery phase is effective in MFG-E8 knockout mice with DSS-induced colitis. In addition, we demonstrated that treatment of WT mice with recombinant MFG-E8 during the recovery phase also enhances repair of intestinal mucosa. Collectively, our data in conjunction with previous findings strongly suggest that MFG-E8 is a crucial protein not only for protection against colitis but also for healing of colitic mucosa.
Several lines of evidence support the role of inflammation in regulation of MFG-E8 gene expression in vivo. For example, MFG-E8 levels are revealed to decrease in numerous tissues during acute inflammation (24). We found that polymicrobial sepsis-induced intestinal injury is associated with downregulation of MFG-E8 gene expression (18). On the other hand, Atabai et al. recently reported that MFG-E8 expression is increased after pulmonary injury induced by bleomycin, a chemotherapeutic agent (25). In the present study, we examined whether intestinal MFG-E8 gene expression is altered during colitis by using a classic experimental colitis model. We found that MFG-E8 gene is expressed constitutively in both colon and rectum in mice. Increased expression of MFG-E8 in both colon and rectum is revealed during the induction of inflammation with DSS treatment. Higher levels of MFG-E8 are revealed to persist in the rectum as compared with the colon on day 7. The MFG-E8 levels in both tissues decrease to below baseline during the water recovery phase in mice with colitis. The alteration of MFG-E8 gene expression is correlated to the levels of inflammatory response and crypt-epithelial injury in both colonic and rectal mucosa in WT mice. Furthermore, administration of MFG-E8 during the recovery phase of colitis attenuates inflammation and improves epithelial repair. Taken together, our novel findings, in combination with previous reports, suggest that maintenance of MFG-E8 level in the intestinal mucosa is critical for tissue repair from colitis.
Aziz et al. previously reported that MFG-E8 is downregulated in the colon and rectum during the acute phase of colitis (corresponding to the DSS induction period), while it gradually became up-regulated during the healing phase when DSS was no longer added to the drinking water in BALB/c mice (19). Clearly, their findings differ from what we observed in C57BL/6J mice in the present study. By comparing data obtained from the inbred C57BL/6J mouse strain in our study to results from BALB/c mice reported by other investigators, we speculated that different inbred mouse strains exhibit differences in alteration of MFG-E8 gene expression during colitis. However, it remains to be determined how genetic background influences MFG-E8 gene expression during the inflammation.
In addition, we show here that MFG-E8 gene expression is increased in the first week of DSS treatment, which may play an important role in protecting the colonic and rectal mucosa from histological damage. Increased expression could be due to increased influx of macrophages into the tissue during DSS treatment. Decrease in the MFG-E8 levels corresponds to increased level of histological damage in the recovery phase. Therapeutic treatment with recombinant MFG-E8 during the recovery phase of DSS colitis promotes intestinal epithelial repair and attenuates inflammation in the intestinal mucosa. Given the established role of MFG-E8 in the maintenance of homeostasis of intestinal epithelium as demonstrated in the present study as well as previous reports (18,19), restoration of MFG-E8 may represent a novel therapeutic target in IBD.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Podolsky DK. (2002) Inflammatory bowel disease. N. Engl. J. Med. 347:417–29.
Yu Y, Sitaraman S, Gewirtz AT. (2004) Intestinal epithelial cell regulation of mucosal inflammation. Immunol. Res. 29:55–68.
Zhao D, et al. (2006) Ghrelin stimulates interleukin-8 gene expression through protein kinase C-mediated NF-kappaB pathway in human colonic epithelial cells. J. Cell. Biochem. 97:1317–27.
Talero E, Sanchez-Fidalgo S, Ramon CJ, Motilva V. (2006) Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int. Immunopharmacol. 6:1404–12.
Talero E, Sanchez-Fidalgo S, Calvo JR, Motilva V. (2007) Chronic administration of galanin attenuates the TNBS-induced colitis in rats. Regul. Pept. 141:96–104.
Pentney PT, Bubenik GA. (1995) Melatonin reduces the severity of dextran-induced colitis in mice. J. Pineal Res. 19:31–9.
Kojima M, Kangawa K. (2006) Drug insight: the functions of ghrelin and its potential as a multitherapeutic hormone. Nat. Clin. Pract. Endocrinol. Metab. 2:80–8.
Kojima M, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 402:656–60.
Dong WG, et al. (2003) Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J. Gastroenterol. 9:1307–11.
Cuzzocrea S, et al. (2001) Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J. Pineal Res. 30:1–12.
Ashizuka S, et al. (2005) Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides. 26:2610–5.
Abad C, et al. (2003) Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 124:961–71.
Abad C, et al. (2005) cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm. Bowel. Dis. 11:674–84.
Stubbs JD, et al. (1990) cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. U.S.A. 87:8417–21.
Yolken RH, et al. (1992) Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 90:1984–91.
Oshima K, et al. (1999) Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem. Biophys. Res. Commun. 254:522–8.
Wang X, Bu HF, De Plaen IG, Tan XD. (2009) Milk fat globule-EGF factor 8 mRNA expression in rat splanchnic tissues during postnatal development. Intl. J. Clin. Exp. Med. 2:36–40.
Bu HF, et al. (2007) Milk fat globule-EGF factor 8/ lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Invest. 117:3673–83.
Aziz MM, et al. (2009) MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J. Immunol. 182:7222–32.
Ensslin MA, Shur BD. (2003) Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 114:405–17.
Krieglstein CF, et al. (2007) Role of blood- and tissue-associated inducible nitric-oxide synthase in colonic inflammation. Am. J. Pathol. 170:490–6.
Bu HF, et al. (2006) Lysozyme-modified probiotic components protect rats against polymicrobial sepsis: role of macrophages and cathelicidinrelated innate immunity. J. Immunol. 177:8767–76.
Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–8.
Cui T, et al. (2009) Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am. J. Respir. Crit. Care Med. 181:238–46.
Atabai K, et al. (2009) Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Invest. 119:3713–22.
Acknowledgment
This work was supported in part by the Grant R01DK064240 (to X-D Tan) from National Institutes of Health, the Excellence in Academic Medicine Award from Illinois Department of Public Aid (to X-D Tan) and Eloise and Warren Batts Investigator Chair (to X-D Tan). We thank Barry D Shur (Emory University School of Medicine) for MFG-E8 deficient mouse colony.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chogle, A., Bu, HF., Wang, X. et al. Milk Fat Globule-EGF Factor 8 Is a Critical Protein for Healing of Dextran Sodium Sulfate-Induced Acute Colitis in Mice. Mol Med 17, 502–507 (2011). https://doi.org/10.2119/molmed.2010.00074
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2010.00074