Abstract
The transcription of the insulinlike growth factor 1 (igf-1) gene generates three mRNA isoforms, namely IGF-1Ea, IGF-1Eb and IGF-1Ec (or MGF [mechano growth factor]). Herein, we analyzed the expression of IGF-1 isoforms in eutopic and ectopic endometrium (red lesions and endometriotic cysts) of women with endometriosis, and we characterized the actions of a synthetic MGF E-peptide on KLE cells. Our data documented that all three igf-1 gene transcripts are expressed in the stromal cells of the eutopic and ectopic endometrium; however, endometriotic cysts contained significantly lower IGF-1 isoform expression, both at the mRNA and protein level, as was shown using semiquantitative PCR and immunohistochemical methods. In addition, the glandular cells of the eutopic endometrium did not express any of the IGF-1 isoforms; however, the glandular cells of the ectopic endometrium (red lesions) did express the IGF-1Ec at mRNA and protein level. Furthermore, synthetic MGF E-peptide, which comprised the last 24 amino acids of the MGF, stimulated the growth of the KLE cells. Experimental silencing of the type 1 IGF receptor (IGF-1R) and insulin receptor expression of KLE cells (siRNA knock-out methods) did not alter the mitogenic action of the synthetic MGF E-peptide, revealing that MGF E-peptide stimulates the growth of KLE cells via an IGF-1R-independent and insulin receptor-independent mechanism. These data suggest that the IGF-1Ec transcript might generate, apart from mature IGF-1 peptide, another posttrans-lational bioactive product that may have an important role in endometriosis pathophysiology.
Similar content being viewed by others
Introduction
Endometriosis is a common benign gynecological condition that is defined as the presence of endometrial stromal and glandular cells outside the endometrial cavity and has a prevalence of 6–10% in women of reproductive age (1). This prevalence is approximately 20% in women with infertility (2). Despite extensive research and numerous theories proposed, the pathogenesis of endometriosis is yet to be determined, and several immunological and growth factors have been investigated in the establishment and maintenance of endometriotic lesions.
In addition, it has been suggested that ectopic endometrial cells undergo decreased apoptosis compared with eutopic endometrial cells (3) and that insulinlike growth factor 1 (IGF-1) is one of the contributing factors that inhibits apoptosis and acts mitogenically on endometrial cells in vitro (4). Furthermore, increased levels of IGF-1 have been found in the peritoneal fluid of women with endometriosis compared with controls (5) and were associated with increased proteolysis of IGF-binding protein 3 (IGFBP-3) mediated by urokinase-type plasminogen activator (uPA) (6,7).
The igf-1 gene contains six exons that, in humans, give rise to three igf-1 gene transcripts by alternative splicing, namely IGF-1Ea, IGF-1Eb and IGF-1Ec (which has also been named mechano growth factor [MGF]). The resulting IGF-1 isoforms undergo posttranslational cleavage to produce a common biologically active product, namely the mature IGF-1, which is encoded by exons 3 and 4, and it is responsible for binding with the IGF receptors and different E-domain products (Figure 1), which contain different parts of exon 5 and/or exon 6 (8–11) and have been proposed to act autonomously (8,12).
Human IGF-1 alternative splicing and encoded propeptides. The igf-1 gene gives rise to multiple mRNA transcripts by alternative splicing. The different IGF-1 mRNA transcripts encode several precursor proteins, which differ by the length of the aminoterminal (signal) peptide and the structure of the extension peptide (E-peptide) on the carboxy-terminal end. The mature IGF-1 peptide results from posttranslational cleavage of all precursor polypeptides, by which the signal and the E-peptide are removed. Exons 5 and 6 encode distinct portions of the E-peptide (called the E-domain) with alternative carboxy-terminal sequences of the extension peptide. The IGF-1Ec splice variant is an exon 4-5-6 variant that produces an E-peptide, termed Ec-peptide. The synthetic MGF E-peptide that comprises the last 24 C-terminal amino acids (aa) of Ec-peptide is shown.
IGF-1 mediates its actions through binding to specific receptors, such as the type 1 IGF receptor (IGF-1R), the insulin receptor (IR), and several atypical receptors such as the hybrid IR/IGF-1R. IGF-1R and IR are cell surface heterotetrameric tyrosine kinase receptors that are coupled to intracellular signaling pathways, such as the ras-raf-MAPK-ERKs and PI3K-AKT signaling cascades (13).
Except for binding IGF-1, IGF-1R can also bind insulinlike growth factor 2 (IGF-2). This is a small peptide that shares approximately 60% of amino acids with IGF-1 and 40% with pro-insulin, and by its binding to IGF-1R, IGF-2 regulates cell proliferation, survival and differentiation. The affinity of IGF-2 for binding IGF-1R is far less than IGF-1 and so it is for insulin (14,15). Although IGF-2 can bind all three receptors (IGF-1R, IGF-2R and IR), its mitogenic and metabolic actions are mediated primarily by binding to IGF-1R. In contrast to IGF-1R, IGF-2R is a transmembrane single-chain glycoprotein known as the cation-independent mannose-6-phosphate receptor (16).
The distinctive biological roles of the IGF-1 isoforms and the mechanisms that regulate their expression have not been clearly documented. Several studies have investigated the expression patterns of these IGF-1 transcripts in skeletal muscle (17–19), and there is growing interest vis-à-vis the potential role of MGF expression in skeletal and cardiac muscle regeneration and hypertrophy after exercise-induced skeletal muscle damage (20) and myocardial infarction (21,22). In addition, we have previously reported preliminary data on the expression of IGF-1 isoforms in endometriosis at mRNA level (23). However, there is little information regarding the IGF-1Ec (MGF) expression in stromal and glandular epithelium of endometriotic lesions and the characterization of the intracellular signaling of MGF E-domain vis-à-vis IGF-1 signaling in endometrial-like cells (20,22).
Herein, we report that all IGF-1 isoforms are expressed in both eutopic and ectopic endometrium, which is, however, significantly lower in endometriotic cysts compared with either eutopic endometrium or red lesions. In addition, we report for the first time that the glandular cells of eutopic endometrium and endometriotic cysts are deprived of any expression of the IGF-1 isoforms, whereas the glandular cells of red lesions express the IGF-1Ec isoform. Furthermore, our data documented that a synthetic MGF E-peptide can stimulate the proliferation of human KLE cells, an endometrial carcinoma cell line with a phenotype of endometrial-like cells, via an IGF-1R-independent and IR-independent mechanism. These data suggest that a posttranslational bioactive E-peptide of the IGF-1Ec isoform may be involved in the pathophysiology of endometriosis.
Materials and Methods
Ethical Approval
A written informed consent was obtained by all the volunteers to participate in this study, which was approved by the Ethics Committee of the National and Kapodistrian University of Athens, and all experimental procedures conformed to the Declaration of Helsinki.
Subjects
The subjects were women of reproductive age undergoing laparoscopy for endometriosis. Median age of the women was 35.7 years (range 28–49), and none had received any form of hormone therapy up to 3 months before the operation. Laparoscopy was performed during the proliferative phase of the menstrual cycle (fifth to tenth day after menstruation).
Tissue Sampling
Tissue sampling was from normal endometrium (eutopic), red lesions and/or endometriotic cysts. We analyzed 15 tissue biopsies of endometriotic peritoneal lesions (red lesions) and 20 tissue biopsies of endometriotic cysts from 15 and 20 patients, respectively. From the same women, normal endometrium was aspirated using the Cornier device (Laboratoire C.C.D., Paris, France). All patients had stage III-IV endometriosis according to revised American Fertility Society (rAFS) classification. Tissue biopsies for RNA and protein extraction were snapfrozen in liquid nitrogen and then stored at −80°C until analysis, whereas biopsies for immunohistochemistry were transferred to formaldehyde 9%. The diagnosis of endometriosis was confirmed with histological examination of related tissue biopsies. The proliferative phase of the menstrual phase was determined based on the last menstrual period and confirmed with histological examination of the eutopic endometrium using the Noyes’ criteria (24).
RNA Extraction and Relative Quantitative PCR Analysis
The expression of IGF-1 transcripts in eutopic and ectopic endometrium (red lesions and/or endometriotic cysts) and in KLE endometrial-like cells was assessed as previously described (23). Briefly, each endometriotic tissue sample was homogenized and total RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. The RNA samples were used for the determination of the mRNA of specific IGF-1 transcripts by reverse transcription (RT) and semiquantitative RT-polymerase chain reaction (PCR) procedures. Both these RT and PCR methods have been described and extensively validated elsewhere (19). Primer sets and PCR conditions used for the assessment of IGF-1 transcripts have been described elsewhere (23).
Protein Extraction and Western Analysis of IGF-1 and MGF
The extracts were analyzed for total protein concentration using the Bradford procedure (Bio-Rad Protein Assay; BioRad, Hercules, CA, USA). Samples were stored in aliquots at −80°C until Western blot analysis as previously described (25). The following primary antibodies were used for the immunodetection of IGF-1Ec (MGF) and IGF-1: MGF, a rabbit antihuman MGF polyclonal antibody (1:10,000 dilution), which was raised against a synthetic peptide corresponding to the last 24 amino acids of the E-domain of human MGF (IGF-1Ec) and characterized in our laboratory, as has been described elsewhere (22); and IGF-1, a mouse monoclonal anti-IGF-1 (1:1,000 dilution) (MS-1508; Thermo Scientific, Fremont, CA, USA; molecular weight of antigen: ~7.6 kDa). After the overnight incubation of blots with the primary antibodies, membranes were incubated with a horseradish peroxidase-conjugated secondary antirabbit IgG (goat antirabbit, 1:2,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or antimouse IgG goat antimouse (1:2,000 dilution; Santa Cruz Biotechnology) for 1 h at room temperature. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to correct for potential variation in the protein loading and to normalize the protein measurements on the same immunoblot. Blots were incubated with a mouse monoclonal primary antibody for GAPDH (1:2,000 dilution; Santa Cruz Biotechnology) and with a horseradish peroxidase-conjugated secondary antimouse IgG (goat antimouse, 1:2,000 dilution; Santa Cruz Biotechnology), and specific band(s) were visualized as described elsewhere (20).
Immunohistochemical Analysis
Formaldehyde-fixed eutopic and ectopic endometrium (red lesions and endometriotic cysts) samples from all patients’ biopsies were paraffin wax embedded and processed for paraffin sections. The sections were incubated with the same primary antibodies used for the Western blot analyses (i.e., the polyclonal anti-MGF antibody at a dilution of 1:1,000 in phosphate-buffered saline (PBS) and the monoclonal anti-IGF-1) (1:50 dilution, MS-1508; Thermo Scientific) overnight at 4°C. After repeated PBS buffer washing, secondary biotinylated goat antirabbit IgG or goat antimouse IgG (DAB; Dako Real EnVision, Glostrup, Denmark) antibody was added for 25 min at room temperature, followed again by repeated PBS buffer washes. Visualization of the immunocomplex was obtained by incubating the sections in a solution of 3,3-diaminobenzidine (DAB) in PBS for 10 min. Tissue sections were visualized under light microscopy, and images were captured on a PENTAX ASAHI digital color camera mounted on the microscope. A qualitative analysis of the tissue sections was then performed in the form of positive or negative staining. Negative control staining procedures were included in all immunohistochemical analyses, as described elsewhere (25).
Cell Cultures
Human KLE cells were obtained by the American Type Culture Collection (ATCC, Bethesda, MD, USA) and maintained as subconfluent monolayers in culture using Dulbecco’s modified Eagle’s medium (DMEM/F-12; Cambrex, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37°C in a humidified atmosphere with 5% CO2, with culture media being replaced every 2–3 d. KLE cells were treated with 0.5 ng/mL up to 30 ng/mL insulin (Novo Nordisk, Bagsværd, Denmark), with 0.5 ng/mL up to 50 ng/mL of mature IGF-1 peptide (rhIGF-1; Chemicon, Temecula, CA, USA) and with 0.5 ng/mL up to 50 ng/mL of a synthetic MGF peptide (which comprises the last 24 amino acids of the E-domain of human MGF, synthesized and validated as previously described [25]; see Figure 1) in a time-dependent manner (i.e., for 24 and 48 h).
Trypan Blue Assay
KLE cells were plated at a cell density of about 2.3 × 104 cell/well in 24-well plates and grown with DMEM/F-12 containing 10% FBS. Twenty-four h after plating, the media were changed to DMEM/F-12 containing 0.5% FBS, and mitogens under investigation were added in a dose-dependent manner (mature IGF-1, MGF E-peptide and insulin). The actual living KLE cell number was measured at different time intervals (24 and 48 h) using the Trypan Blue exclusion assays, as previously described (26).
IGF-1R and IR siRNA Knock Out
To investigate if the synthetic MGF E-peptide acts on KLE cells via the IGF-1R-or IR-mediated pathway, IGF-1R and IR expression was silenced in KLE cells using the commercially available Stealth siRNA technology (Invitrogen). Three different 25-mer siRNA molecules were examined in each case for their potential to knock out (KO) the expression of IR and that of IGF-1R in KLE cells. It was determined that the most efficient KO of the IR was obtained by using the ACAAACUGCCCGUUGAUGACGGUGG siRNA duplex at a concentration of 40 pmol by using the reverse transfection method. In the case of IGF-1R KO, the molecule of choice was the UCUUC AAGGGCAAUUUGCUCAUUAA siRNA duplex, at a concentration of 50 pmol, again by using reverse transfection according to the manufacturer’s instructions. As a negative control, we used a universal negative control stealth siRNA (Invitrogen). In brief, KLE cells were grown in 10% DMEM/F-12 media. The transfection mixture was obtained by diluting the 40 pmol of the siRNA duplex in 100 µL OptiMem serum-free medium (Invitrogen) in a well of a 24-well plate, followed by the addition of 2 µL lipofectamine RNAiMAX (Invitrogen). After 20 min, 500 µL of the trypsinized KLE cells was added to the mixture. Forty-eight hours after the KO, the media switched to DMEM 0.5% FBS, and after 24 h, the IR KO cells were exposed to either insulin or MGF E-peptide, whereas the IGF-1R KO cells were exposed to mature IGF-1 or MGF E-peptide for 24 and 48 h in triplicate determinations. The viable cells were counted using the Trypan Blue exclusion assay.
Expression of IGF-1R and IR
The expression level of IGF-1R and IR transcripts, after the siRNA IGF-1R KO and IR KO in KLE cells, was assessed by quantitative real-time PCR (qRT-PCR). The KO mRNA levels were determined 48 h after the siRNA KO according to the manufacturer’s instructions. As an internal control, we used GAPDH and β-actin. The validation of the product identity was obtained by the melting curve. Quantitative RT-PCR to examine the levels of expression of IGF-1R and IR was carried out before and after the siRNA IGF-1R or IR KO in KLE cells. Briefly, RT-PCR data quantification analysis was carried out in the forms of melting and amplification curves, cycle threshold (Ct) values and normalized gene expression (Delta Delta Ct [ddCt]), using the Bio-Rad IQ5 optical software 2.0. The primers used in the reactions were generated using the FastPCR program and were as follows: IGF-1R forward: ACCCGGAGTACTTCAGCGC; IGF-1R reverse: CACAGAAGCTTCGTTGAGAA; IR forward: ACTCTCAGATCCTGA AGGAGCTGGA; IR reverse: AGTGT TGGGGAAAGCTGCCAC. The set of primers for IR was designed to detect both IR isoforms in a single PCR. The PCR conditions were the same in both cases: 95°C for 30 s × 1 cycle, 94°C for 20 s, 60°C for 30 s, 72°C for 30 s × 35 cycles and 72°C for 5 min.
Statistical Analysis
Changes in cell numbers were assessed using analysis of variance (ANOVA) (SPSS v. 11 statistical package; SPSS, Chicago, IL, USA). Where significant F ratios were found (P < 0.05), the means were compared using Tukey post hoc tests. A Student t test was used to evaluate transcriptional and translational differences in IGF-1 isoform expression between eutopic endometrium and endometriotic cysts or red lesions. All data are presented as mean ± SD. The level of significance was set at P < 0.05.
Results
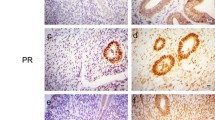
The expression of IGF-1 mRNA transcripts was found to be significantly lower in the endometriotic cysts compared with that of the eutopic endometrium and that of the red lesions, as assessed by semiquantitative PCR methods (Figure 2). Similar patterns of the IGF-1 transcripts translation were also detected in red lesions and endometriotic cysts compared with eutopic endometrium at the protein level (Figure 3A, B). The immunohistochemical analysis revealed that in the eutopic endometrium (Figure 4A, E) and the endometriotic cysts (Figure 4C, G), the IGF-1 transcripts were expressed only in the stromal cells and not in the glandular epithelium, whereas in the red lesions, the IGF-1 transcripts were expressed not only in the stroma but also in the glandular cells (Figure 4B, F). All 15 red lesion biopsies were positive for glandular MGF/IGF-1 expression, whereas all 15 biopsies from the eutopic endometrium (of the same women) were negative for glandular MGF/IGF-1 expression. The stroma was steady positive for MGF/IGF-1 expression in all eutopic and ectopic endometrial biopsies; however, endometriotic cysts did express considerably lower MGF/IGF-1 levels, as noted by immunohistochemical analysis.
Expression of the different IGF-1 transcripts (IGF-1 Ea, IGF-1 Eb and IGF-1 Ec [MGF]) in eutopic endometrium (EU), peritoneal red lesion (PE) and ovarian endometriotic cyst (OvE). Representative PCR gel images demonstrate the differential mRNA expression of the IGF-1 transcripts in PE and in OvE compared with EU (upper panel). In the lower panel, PCR relative quantification is presented. Values of PE and OvE were normalized to each corresponding ribosomal 18S and expressed as percentage differences (%) from EU levels (means ± SD, PE: n = 15, OvE: n = 20). *Significantly different from EU (P< 0.01).
(A–D) Cytoplasmic localization of IGF-1 in stromal cells (SC) in eutopic endometrium (A), endometriotic lesion (B) and endometriotic cyst (C). Note the absence of staining in glandular epithelium (GE) in eutopic endometrium as opposed to the positive staining of glandular epithelium in endometriotic lesions. (D) Negative control. (E-H) Cytoplasmic localization of MGF (IGF-1Ec) in stromal cells (SC) of tissue biopsies from eutopic endometrium (E), endometriotic lesion (F) and endometriotic cyst biopsies (G). Note the absence of MGF staining in glandular epithelium biopsies (GE) of the eutopic endometrium and endometriotic cyst as opposed to the positive staining of glandular epithelium in endometriotic lesion. (H) Negative control. Solid arrows represent stromal cells; hollow arrows represent glandular epithelium.
In order to characterize the IGF-1Ec posttranslational products (mature IGF-1 peptide and synthetic MGF E-peptide) in vitro, we initially characterized the KLE cells. We documented that the KLE endometrial-like cells express all three IGF-1 mRNA transcripts, which are certainly translated to pro-IGF-1 and pro-IGF-1Ec (MGF) products at protein level (Figure 5A, B).
Expression of the different igf-1 gene transcripts in KLE endometrial-like cells. (A) PCR products (that is, amplified target cDNAs) from the different primer sets and PCR conditions used for the detection of IGF-1 transcripts at the mRNA level. An equal amount of each PCR product was loaded onto a 2% agarose gel and separated by electrophoresis. (B) Translational products of the different IGF-1 mRNA transcripts were detected by Western blot analysis using antibodies specific for anti-IGF-1 and anti-IGF-1Ec (MGF).
Because the actions of IGF-1 can be mediated not only via its high-affinity IGF-1R but also via IRs as well as hybrid IGF-1R/IR, we experimentally engineered KLE cells with silenced IGF-1R and IR expression, using siRNA methods, to further characterize MGF E-peptide actions in KLE cells. Thus, we generated transfectans of siRNA IGF-1R KO KLE cells and siRNA IR KO KLE cells. Indeed, we achieved approximately 60–80% reduction of IR mRNA expression (for both IR transcripts; IR-A and IR-B) compared with the respective expression levels assessed in control KLE cells (Figure 6A, D, G). Similar results were obtained in the siRNA IGF-1R KLE cells (Figure 6B, E, F). Analyses of β-actin (Figure 6C) and GAPDH expression (not shown) were used as internal controls for normalization in all cases.
Characterization of the degree of reduction of IR expression in IR KO KLE cells (A) and of IGF-1R expression in IGF-1R KO KLE cells (B) by qRT-PCR. IR KO and IGF-1R KO lines represent the melting curves of IR (IR-A and IR-B isoforms) and IGF-1R in IR KO KLE and IGF-1R KO KLE cells, respectively, compared with the lines of IR and IGF-1R in control KLE cells. The amplification curves and Cts (D, E) as well as normalized expression (ddCt) charts (F, G) are also shown. The degree of reduction of IR and IGF-1R expression was from 60% up to 80% in this cell line. Normalization in all the cases was carried out by β-actin (C).
Using these KLE transfectans, we were able to show that exogenous IGF-1 and insulin administration did not stimulate the IGF-1R KO and IR KO KLE cells. On the contrary, IGF-1 and insulin stimulated the growth of parental KLE cells (Table 1). Interestingly, MGF E-peptide stimulated the proliferation of parental and IGF-1R KO and IR KO KLE cells (see Table 1).
Discussion
In this study, we documented that eutopic and ectopic (endometriotic cysts and red lesions) endometrium obtained from women with endometriosis as well as human KLE endometrial-like cells express IGF-1 transcripts. We have defined that the IGF-1Ec transcript is expressed both at the mRNA and protein level. This particular IGF-1 transcript has been associated with regeneration mechanisms of skeletal muscle and myocardial cells (20–22).
Semiquantitative analysis of the IGF-1 transcript expression using PCR methods revealed that endometriotic cysts expressed IGF-1 transcripts at a significantly lower level than eutopic endometrium and red lesions. These findings were inline with our preliminary data previously published (23). Our findings could be explained by the fact that even though endometriotic cysts represent a feature of advanced disease, they are characterized by the presence of fibrosis and low levels of active endometriotic tissue. This result is consistent with the natural history of the disease, during which active endometriotic tissue is substituted by fibrotic tissue accounting for the increased scarring and adhesion formation found in late stages of endometriosis (23). This result is also consistent with the results of our previous studies, where we documented increased expression of other components of the IGF bioregulatory system, which includes IGFs/uPA/plas-min/IGFBP-3 expression (6,7,27).
In this study, we used specific antibodies to identify the expression of IGF-1 and MGF in endometriotic biopsies by immunohistochemical and Western blot analyses. Because IGF-1 peptide is a common product of all three IGF-1 transcripts, anti-IGF-1 antibody can detect the expression of pro-IGF-1 peptide from any IGF-1 transcript. On the contrary, our anti-MGF antibody identifies the expression of the IGF-1Ec (MGF) transcript only. The immunohistochemical analysis of IGF-1 transcripts posttranslational products revealed that in eutopic endometrium and endometriotic cysts, IGF-1 and IGF-1Ec (MGF) were expressed only in stroma cells but not in glandular cells. In contrast, in red lesions, there was positive staining not only in stroma cells but also in glandular epithelium. Even though histological diagnosis of endometriosis requires the presence of stroma and glandular cells in tissue biopsies, the proportion of stroma/glands in endometriotic tissue is not constant, and it has been suggested that lesions related to more active forms of endometriosis (for example, red lesions) present a higher proportion of glandular cells (28). This was evident in our biopsies as well, as histological examination showed increased proportion of glandular epithelium in red lesions compared with endometriotic cysts. This could account for the increased IGF-1 transcripts expression (although not significant) in red lesions as it was documented by semiquantitative PCR analysis in our study. The expression of IGF-1 and IGF-1Ec in the glandular epithelium of only endometriotic lesions and not in eutopic endometrium and endometriotic cysts could favor our hypothesis that IGF-1 and IGF-1Ec isoforms are associated with active endometriosis, and their action in ectopic endometriotic cells could be involved in the progression of the disease and evolution of endometriotic lesions.
The IGF-1 stimulates the growth and differential function of endometrial cells via the IGF-1R, and possibly via several atypical receptors, including the hybrid IR/IGF-1R. The latter is composed of an IR hemi-receptor linked to an IGF-IR hemi-receptor and has been reported to have an important role in cancer biology (29–31). Recently, the two IR isoforms (IR-A and IR-B) have been reported that are overexpressed in cancer tissues (32), whereas the expression of IGF-1R has been previously characterized in KLE cells in our laboratory (7). Therefore, aiming to the characterize the MGF E-peptide actions in KLE cells, we performed a series of silencing experiments of these major receptors involved in the IGF-mediated actions. Our data suggested that silencing of the IGF-1R and IR expression in KLE cells did not have an important effect on the proliferative activity of the exogenous MGF E-peptide in vitro, thus suggesting that synthetic MGF E-peptide action is apparently mediated via an IGF-1R-independent, IR-independent mechanism. Because the IR/IGF-1R hybrid receptor consists of IR and IGF-1R hemi-re-ceptors, the silencing of the IR or the IGF-1R is expected to block the formation of the hybrid receptor. Therefore, our experiments suggested that mitogenic activity of the synthetic MGF E-peptide is mediated via another receptor molecule.
Further evidence for such autonomous actions of the synthetic MGF E-peptide was provided by our recent data, which revealed that MGF E-peptide activated ERK1/2 phosphorylation but did not activate AKT phosphorylation in skeletal muscle-like and myocardial-like cells (20,22). This particular phosphorylation pattern generated by the MGF E-peptide is in agreement with the trypan blue exclusion assays in KLE cells, thus suggesting that MGF E-peptide activity is via an IGFR/IR-independent mechanism and via an as yet unidentified molecule.
In conclusion, our data suggest the possible role of IGF-1Ec (MGF) expression in endometriosis. This is supported by the preferential expression of this IGF-1 transcript in glandular epithelial cells in ectopic endometrium only (red lesions). Conceivably, this preferential MGF expression generates posttranslational products IGF-1 and MGF E-peptide, with the latter being capable of stimulating the proliferation of endometrial-like cells via an IGF-1R-independent, IR-independent and hybrid IGF-1R/IR-independent mechanism. These data suggest that there may be a role for MGF in the pathogenesis of endometriosis that is autonomous and independent from the IGF system.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Giudice LC, Kao LC. (2004) Endometriosis. Lancet 364:1789–99.
Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. (2006) Economic burden of endometriosis. Fertil. Steril. 86:1561–72.
Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. (1998) Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil. Steril. 69:1042–7.
Koutsilieris M, Mastrogamvrakis G, Lembessis P, Sourla A, Miligos S, Michalas S. (2001) Increased insulin-like growth factor 1 activity can rescue KLE endometrial-like cells from apoptosis. Mol. Med. 7:20–6.
Kim JG, Suh CS, Kim SH, Choi YM, Moon SY, Lee JY. (2000) Insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal fluid of patients with and without endometriosis. Fertil. Steril. 73:996–1000.
Koutsilieris M, Lavergne E, Lemay A. (1997) Association of protease activity against IGFBP-3 with peritoneal fluid mitogens: possible implications for the ectopic growth of endometrial cells in women with endometriosis. Anticancer Res. 17:1239–44.
Lembessis P, Kalariti N, Koutsilieris M. (2004) Glucocorticoid receptor function suppresses insulin-like growth factor 1 activity in human KLE endometrial-like cells. In Vivo 18:43–7.
Siegfried JM, Kasprzyk PG, Treston AM, Mulshine JL, Quinn KA, Cuttitta F. (1992) A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc. Natl. Acad. Sci. U. S. A. 89:8107–11.
Gilmour RS. (1994) The implications of insulinlike growth factor mRNA heterogeneity. J. Endocrinol. 140:1–3.
Chew SL, Lavender P, Clark AJ, Ross RJ. (1995) An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology 136:1939–44.
Shavlakadze T, Winn N, Rosenthal N, Grounds MD. (2005) Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm. IGF Res. 15:4–18.
Kuo YH, Chen TT. (2002) Novel activities of pro-IGF-I E peptides: regulation of morphological differentiation and anchorage-independent growth in human neuroblastoma cells. Exp. Cell Res. 280:75–89
Samani AA, Yakar S, LeRoith D, Brodt P. (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 28:20–47.
Jones JI, Clemmons DR. (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 16:3–34.
LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. (1995) Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 16:143–63.
Kornfeld S. (1992) Structure and function of the mannose-6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61:307–30.
Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J. Physiol. 547:247–54.
Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. (2005) Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J. Appl. Physiol. 98:482–8.
Kim JS, Cross JM, Bamman MM. (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am. J. Physiol. Endocrinol. Metab. 288:E1110–9.
Philippou A, et al. (2009) Expression of IGF-1 iso-forms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro. In Vivo 23:567–75.
Carpenter V, et al. (2008) Mechano-growth factor reduces loss of cardiac function in acute myocardial infarction. Heart Lung Circ. 17:33–9.
Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M. (2009) IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol. Med. 15:127–35.
Milingos D, et al. (2006) Insulin-like growth factor-1 isoform mRNA expression in women with endometriosis: eutopic endometrium versus endometriotic cyst. Ann. N. Y. Acad. Sci. 1092:434–9.
Noyes RW, Hertig AT, Rock J. (1975) Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 122:262–3.
Philippou A, Stavropoulou A, Sourla A, Pissimissis N, Halapas A, Maridaki M, Koutsilieris M. (2008) Characterization of a rabbit antihuman mechano growth factor (MGF) polyclonal antibody against the last 24 amino acids of the E domain. In Vivo 22:27–35.
Koutsilieris M, Rabbani SA, Goltzman D. (1987) Effects of human prostatic mitogens on rat bone cells and fibroblasts. J. Endocrinol. 115:447–54.
Koutsilieris M, Sourla A, Pelletier G, Doillon CJ. (1994) Three-dimensional type I collagen gel system for the study of osteoblastic metastases produced by metastatic prostate cancer. J. Bone Miner. Res. 9:1823–32.
Donnez J, Squifflet J, Casanas-Roux F, Pirard C, Jadoul P, van Langendonckt A. (2003) Typical and subtle atypical presentations of endometriosis. Obstet. Gynecol. Clin. N. Am. 30:83–93.
Bogdanos J, Karamanolakis D, Tenta R, Tsintavis A, Milathianakis C, Mitsiades C, Koutsilieris M. (2003) Endocrine/paracrine/autocrine survival factor activity of bone microenvironment participates in the development of androgen ablation and chemotherapy refractoriness of prostate cancer metastasis in skeleton. Endocr. Relat. Cancer 10:279–89.
Koutsilieris M, Mitsiades C, Sourla A. (2000) Insulin-like growth factor I and urokinase-type plasminogen activator bioregulation system as a survival mechanism of prostate cancer cells in osteoblastic metastases: development of anti-survival factor therapy for hormone-refractory prostate cancer. Mol. Med. 6:251–67.
Koutsilieris M. (1992) Pathophysiology of uterine leiomyomas. Biochem. Cell Biol. 70:273–8.
Cox ME, et al. (2009) Insulin receptor expression by human prostate cancers. Prostate 69:33–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Milingos, D.S., Philippou, A., Armakolas, A. et al. Insulinlike Growth Factor-1Ec (MGF) Expression in Eutopic and Ectopic Endometrium: Characterization of the MGF E-Peptide Actions In Vitro. Mol Med 17, 21–28 (2011). https://doi.org/10.2119/molmed.2010.00043
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2010.00043